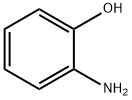
2-аминофенол
- английское имя2-Aminophenol
- CAS №95-55-6
- CBNumberCB8309112
- ФормулаC6H7NO
- мольный вес109.13
- EINECS202-431-1
- номер MDLMFCD00007690
- файл Mol95-55-6.mol
| Температура плавления | 172 °C |
| Температура кипения | 164 °C (11.2515 mmHg) |
| Плотность накопления | 600kg/m3 |
| плотность | 1.328 |
| давление пара | 14 hPa (153 °C) |
| показатель преломления | 1.5444 (estimate) |
| Fp | 168 °C |
| температура хранения | Store below +30°C. |
| растворимость | 17 g/L (20°C) |
| Цветовой индекс | 76520 |
| форма | Powder |
| пка | 4.78, 9.97(at 20℃) |
| цвет | White to brown |
| РН | 6.5-7.5 (10g/l, H2O, 20℃) |
| Водородный показатель | 6.5 - 7.5 at 10 g/l at 20 °C |
| Растворимость в воде | 17 g/L (20 ºC) |
| Мерк | 14,461 |
| БРН | 606075 |
| Стабильность | Stable, but may be air or light sensitive. Incompatible with strong oxidizing agents. |
| LogP | 0.620 |
| Справочник по базе данных CAS | 95-55-6(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 4-6 |
| FDA UNII | 23RH73DZ65 |
| Справочник по химии NIST | Phenol, 2-amino-(95-55-6) |
| Система регистрации веществ EPA | o-Aminophenol (95-55-6) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn,C | |||||||||
| Заявления о рисках | 20/22-68-34-20/21/22 | |||||||||
| Заявления о безопасности | 28-36/37-28A-45-36/37/39-26-68-20/22 | |||||||||
| РИДАДР | UN 2512 6.1/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | SJ4950000 | |||||||||
| F | 8-10-23 | |||||||||
| Температура самовоспламенения | 190 °C | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29222900 | |||||||||
| Банк данных об опасных веществах | 95-55-6(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 1300 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H341:Предполагается, что данное вещество вызывает генетические дефекты.
H302+H332:Вредно при проглатывании или при вдыхании.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P301+P312+P330:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии. Прополоскать рот.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
2-аминофенол химические свойства, назначение, производство
Химические свойства
2-aminophenol appears as colorless needles or as white crystalline substance turning tan to brown on exposure to air. It forms white orthorhombic bipyramidal needles when crystallized from water or benzene. The crystals have eight molecules to the elementary cell. Insoluble in benzene, soluble in ethanol and water. o-aminophenol is contained in hair dyes and can cause contact dermatitis in hairdressers.Использование
2-Aminophenol is an isomer of 4-Aminophenol and is used as a reagent for the synthesis of heterocyclic compounds and dyes. It is also used to make dyes and pharmaceuticals.Определение
ChEBI: 2-aminophenol is the aminophenol which has the single amino substituent located ortho to the phenolic -OH group. It has a role as a bacterial metabolite.Подготовка
2-Aminophenol is obtained by reduction of o-nitrophenol with sodium sulfide or hydrogen gas.Антимикробная активность
Further observations on this group of compounds are required, but its low toxicity and its bacteriostatic activity against the Gram-negative bacilli suggest that 2-aminophenol may be of value as a local antiseptic.Общее описание
2-Aminophenol, also called o-aminophenol, is an aromatic amphoteric compound with the formula C6H4(OH)NH2. It is a white needle-shaped crystal that darkens when exposed to light and air. It is an isomer of aminophenol and serves as a vital chemical intermediate for dyes, medicine, printing, and biological applications.Реакции воздуха и воды
Protect from air and light. Insoluble in water.Профиль реактивности
2-Aminophenol can react with oxidizing agents. THF forms explosive products with 2-aminophenol [Lewis 3227].Пожароопасность
Combustible material: may burn but does not ignite readily. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.Контактные аллергены
It is contained in hair dyes and can cause contact dermatitis in hairdressers and consumersПобочные эффекты
(1)Methemoglobinemia - an increase in methemoglobin in the blood; the compound is classified as having secondary toxic effects.(2)Skin sensitiser - a substance that can induce an allergic skin reaction.
(3)Asthma - reversible bronchoconstriction (narrowing of the fine bronchial tubes) caused by inhalation of irritating or allergenic substances.
Профиль безопасности
Poison by intraperitoneal and subcutaneous routes. Moderately toxic by ingestion. An experimental teratogen. Other experimental reproductive effects. An eye irritant. Mutation data reported. When heated to decomposition it emits toxic NO,. See also AROMATIC AMINES.Возможный контакт
Workers may be exposed to oAminophenol during its use as a chemical intermediate; in the manufacture of azo and sulfur dyes; and in the photographic industry. There is potential for consumer exposure to o-Aminophenol because of its use in dyeing hair, fur, and leather. The compound is a constituent of 75 registered cosmetic products suggesting the potential for widespread consumer exposure. p-Aminophenol is used mainly as a dye, dye intermediate and as a photographic developer; and in small quantities in analgesic drug preparation. Consumer exposure to p-aminophenol may occur from use as a hairdye or as a component in cosmetic preparations. mAminophenol is used mainly as a dye intermediateПеревозки
UN2512 Aminophenols (o-; m-; p-), Hazard Class: 6.1; Labels: 6.1-Poisonous materialsМетоды очистки
Purify it by dissolving it in hot water, decolorising with activated charcoal, filtering and cooling to induce crystallisation. Maintain an atmosphere of N2 over the hot phenol solution to prevent its oxidation [Charles & Freiser J Am Chem Soc 74 1385 1952]. It can also be crystallised from EtOH using the same precautions. [Beilstein 13 IV 805.]Несовместимости
These phenol/cresol materials can react with oxidizers; reaction may be violent. Incompatible with strong reducing substances such as alkali metals, hydrides, nitrides, and sulfides. Flammable gas (H2) may be generated, and the heat of the reaction may cause the gas to ignite and explode. Heat may be generated by the acidbase reaction with bases; such heating may initiate polymerization of the organic compound. Reacts with boranes, alkalies, aliphatic amines, amides, nitric acid, sulfuric acid. Phenols are sulfonated very readily (e.g., by concentrated sulfuric acid at room temperature). These reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid and can explode when heated. Many phenols form metal salts that may be detonated by mild shock.Утилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.2-аминофенол запасные части и сырье
2-аминофенол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +862156762820 +86-13564624040 |
China | 7707 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-021-62885108 +8613917661608 |
China | 2068 | 57 | |
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +86-371-66670886 | China | 19902 | 58 | |
| 0411-84820922 8613904096939 |
China | 304 | 57 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 008657128800458; +8615858145714 |
China | 7738 | 55 |