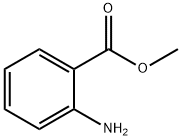
МЕТИЛАНТРАНИЛАТ
- английское имяMethyl anthranilate
- CAS №134-20-3
- CBNumberCB0722306
- ФормулаC8H9NO2
- мольный вес151.16
- EINECS205-132-4
- номер MDLMFCD00007710
- файл Mol134-20-3.mol
| Температура плавления | 24 °C (lit.) |
| Температура кипения | 256 °C (lit.) |
| плотность | 1.168 g/mL at 25 °C (lit.) |
| давление пара | 1 mm Hg ( 20 °C) |
| показатель преломления | n |
| FEMA | 2682 | METHYL ANTHRANILATE |
| Fp | 220 °F |
| температура хранения | Keep in dark place,Inert atmosphere,Room temperature |
| растворимость | alcohol: freely soluble(lit.) |
| пка | pK1:2.23(+1) (25°C) |
| форма | Liquid |
| цвет | Clear yellow-brown |
| РН | 7.5-8 (H2O, 20℃)Aqueous solution |
| Запах | grape odor |
| Odor Type | fruity |
| Биологические источники | synthetic |
| Вязкость | 7.14mm2/s |
| Пределы взрываемости | 1.4-7.8%(V) |
| Растворимость в воде | slightly soluble |
| Чувствительный | Air Sensitive |
| Мерк | 14,6020 |
| Номер JECFA | 1534 |
| БРН | 606965 |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents. |
| ИнЧИКей | VAMXMNNIEUEQDV-UHFFFAOYSA-N |
| LogP | 1.88 at 20℃ |
| Вещества, добавляемые в пищу (ранее EAFUS) | METHYL ANTHRANILATE |
| FDA 21 CFR | 182.60; 582.60 |
| Справочник по базе данных CAS | 134-20-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 981I0C1E5W |
| Справочник по химии NIST | Benzoic acid, 2-amino-, methyl ester(134-20-3) |
| Пестициды Закон о свободе информации (FOIA) | Methyl anthranilate |
| Система регистрации веществ EPA | Methyl anthranilate (134-20-3) |
| UNSPSC Code | 12352100 |
| NACRES | NA.22 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/37/38 | |||||||||
| Заявления о безопасности | 26-36-37/39 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | CB3325000 | |||||||||
| F | 21 | |||||||||
| Температура самовоспламенения | 986 °F | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29224995 | |||||||||
| Банк данных об опасных веществах | 134-20-3(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats, mice: 2910, 3900 mg/kg, P. M. Jenner et al., Food Cosmet. Toxicol. 2, 327 (1964) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P337+P313:Если раздражение глаз не проходит обратиться за медицинской помощью.
МЕТИЛАНТРАНИЛАТ химические свойства, назначение, производство
Описание
Methyl anthranilate, also known as MA, methyl 2-amino benzoate or carbo methoxy aniline, is an ester of anthranilic acid. Its chemical formula is C8H9NO2.Химические свойства
Methyl Anthranilate occurs in a large number of blossom essential oils (e.g., neroli, ylang-ylang, and jasmine oils), grapes, and citrus oils. It occurs as white crystals (mp 24–25°C), or a yellowish liquid, that show blue fluorescence and have an orange blossom odor. Methyl anthranilate is prepared by esterification of anthranilic acid with methanol or by reaction of isatoic anhydride with methanol.It is used in a large number of blossom fragrances. However, its use in perfumes for soaps and cosmetics is limited because it causes discoloration. It is used in flavor compositions (e.g., in grape and citrus flavors).
Вхождение
Methyl anthranilate naturally occurs in the Concord grapes and other Vitis labrusca grapes or hybrids thereof, and in bergamot, black locust, champaca , gardenia, jasmine, lemon, mandarin, neroli, oranges, rue oil, strawberry, tuberose, wisteria, galangal and ylang ylang. It is also a primary component of the essential apple flavor, along with ethyl acetate and ethyl butyrate.It is also secreted by the musk glands of foxes and dogs, and lends a "sickly sweetness" to the smell of rotting flesh.Использование
Methyl anthranilate acts as a bird repellent. It is food-grade and can be used to protect corn, sunflowers, rice, fruit, and golf courses. Dimethyl anthranilate (DMA) has a similar effect. It is also used for the flavor of grape Kool Aid. It is used for flavoring of candy, soft drinks (e.g. grape soda), gums, and drugs.Methyl anthranilate both as a component of various natural essential oils and as a synthesised aroma-chemical is used extensively in modern perfumery . It is also used to produce Schiff's Bases with aldehydes, many of which are also used in perfumery. In a perfumery context the most common Schiff's Base is known as aurantiol - produced by combining methyl anthranilate and hydroxyl citronellal.
Подготовка
By heating anthranilic acid and methyl alcohol in the presence of sulfuric acid and subsequent distillation.Определение
ChEBI: Methyl anthranilate is a benzoate ester that is the methyl ester of anthranilic acid. It has a role as a metabolite and a flavouring agent. It derives from an anthranilic acid.Общее описание
Clear colorless to tan liquid with an odor of grapes. Has light blue fluorescence.Реакции воздуха и воды
Methyl anthranilate is sensitive to air and light. Slightly water soluble .Профиль реактивности
An amine and ester. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.Пожароопасность
Methyl anthranilate is combustible.Токсикология
Methyl anthranilate is a colorless liquid that has a sweet, fruity, grape-like flavor. It is found in the essential oils of orange, lemon, and jasmine and has been widely used to create imitation Concord grape flavor. Table 10.8 shows the acute toxicity of methyl anthranilate. Methyl anthranilate promotes some allergic reactions on human skin, which has led to it being prohibited for use in cosmetic products.Профиль безопасности
Moderately toxic by ingestion. Experimental reproductive effects. A skin irritant. See also ESTERS. Combustible liquid. When heated to decomposition it emits toxic fumes of NOx.Безопасность
Methyl anthranilate is a plant-based compound with a long history of use as a flavor additive for foods and beverages, and as an aromatic used extensively in perfumery. As such, the US Department of Agriculture (USDA) and the Food and Drug Administration (FDA) have approved MA as "generally recognized as safe".Метаболизм
It is probable that this ester is hydrolysed and the anthranilate is excreted mostly as oaminobenzoyl glucuronide (Charconnet-Harding, Dalgliesh & Neuberger, 1953).Растворимость в органика
Methyl anthranilate is soluble in ethanol and propylene glycol. It is insoluble in paraffin oil.МЕТИЛАНТРАНИЛАТ запасные части и сырье
сырьё
1of4
запасной предмет
- 2-АМИНО-3,5-ДИБРОМБЕНЗИЛОВЫЙ СПИРТ
- Метиловый эфир 2-амино-3 ,5-dibromobenzoate
- Saccharin sodium dihydrate
- Сахарин
- Methyl 2-(methylamino)benzoate
1of4
МЕТИЛАНТРАНИЛАТ поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0519-8359-8696 +8618018249389 |
China | 9897 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +undefined18602966907 | China | 997 | 58 | ||
| +8619931165850 | China | 1000 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-86-1913198-3935 +8617331935328 |
China | 970 | 58 | ||
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | ||
| +8613343047651 | China | 3692 | 58 | ||
| +86-18633929156 +86-18633929156 |
China | 972 | 58 |