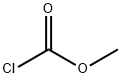
МЕТИЛХЛОРОФОРМАТ
- английское имяMethyl chloroformate
- CAS №79-22-1
- CBNumberCB6854401
- ФормулаC2H3ClO2
- мольный вес94.5
- EINECS201-187-3
- номер MDLMFCD00000639
- файл Mol79-22-1.mol
| Температура плавления | -61°C |
| Температура кипения | 70-72 °C(lit.) |
| плотность | 1.223 g/mL at 25 °C(lit.) |
| плотность пара | 3.26 (vs air) |
| давление пара | 4.8 psi ( 20 °C) |
| показатель преломления | n |
| Fp | 64 °F |
| температура хранения | Store at 0-5°C |
| растворимость | Chloroform, Ethyl Acetate |
| форма | Liquid |
| цвет | Clear colorless to light yellow |
| Запах | Acrid. |
| Растворимость в воде | hydrolysis |
| Мерк | 13,6071 |
| Диэлектрическая постоянная | 12.9 |
| Стабильность | Moisture Sensitive |
| Справочник по базе данных CAS | 79-22-1(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | RC6VA8OB2N |
| Справочник по химии NIST | Carbonochloridic acid, methyl ester(79-22-1) |
| Система регистрации веществ EPA | Methyl chlorocarbonate (79-22-1) |
| Коды опасности | F,T+ | |||||||||
| Заявления о рисках | 11-21/22-26-34 | |||||||||
| Заявления о безопасности | 14-26-28-36/37/39-45-63-46-28A-39-36/37 | |||||||||
| РИДАДР | UN 1238 6.1/PG 1 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | FG3675000 | |||||||||
| F | 10-19-21 | |||||||||
| Температура самовоспламенения | 485 °C | |||||||||
| Класс опасности | 6.1(a) | |||||||||
| Группа упаковки | I | |||||||||
| кода HS | 29151300 | |||||||||
| Банк данных об опасных веществах | 79-22-1(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H330:Смертельно при вдыхании.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H312:Вредно при попадании на кожу.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P310:Немедленно обратиться за медицинской помощью.
МЕТИЛХЛОРОФОРМАТ химические свойства, назначение, производство
Химические свойства
Methyl chloroformate is a colorless liquid with an unpleasant, acrid odor. This is a highly corrosive and flammable material. Decomposed by hot water; stable to cold water. Soluble in methanol alcohol, ether, and benzene.Использование
Chloroformic Acid Methyl Ester is used in the synthesis of a new class of potent Cdk4 inhibitors in the treatment of cancer. Also used in the synthesis of Phorboxazole B.прикладной
Methyl chloroformate (MCF) is generally used for the derivatization of functional groups such as carboxylic acids, amines, and phenols.MCF can also be used:
To activate 3-acylpyridines for nucleophilic addition with alkynyltin reagents to form 2,3-disubstituted 1,2-dihydropyridines.
Chloroesterification of terminal alkynes to form β-chloro-α,β-unsaturated esters.
As an electrophilic reagent to mediate the reaction of pyridine with lithium dialkyl- or diarylcuprates to form 4-substituted 1,4-dihydropyridine derivatives.
To convert nitronates into methoxycarbonyl nitronates.
Методы производства
Prepared from phosgene and methyl alcohol.Общее описание
A colorless liquid with a pungent odor. Flash point 54°F. Corrosive to metals and tissue. Vapors heavier than air. Very toxic by inhalation. Used to make other chemicals and insecticides.Реакции воздуха и воды
Highly flammable. Gives off hydrochloric acid fumes in contact with moist air. Slightly soluble in water and decomposed by water to hydrochloric acid with evolution of heat.Профиль реактивности
Methyl chloroformate is incompatible with water, strong oxidizing agents, alcohols, bases (including amines). Decomposes slowly in water to yield methanol, HCl, and CO2; reaction can be hazardous if water is hot. Attacks many metals especially in humid atmosphere [Handling Chemicals Safely 1980. p. 476]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].Опасность
Flammable, dangerous fire risk. Highly corrosive and irritant to skin and eyes.Угроза здоровью
Methyl chloroformate is highly toxic upon inhalation and upon ingestion. A concentration of 1 mg/liter (190 ppm) has been lethal in 10 minutes. It is corrosive and irritating to skin.Пожароопасность
Methyl chloroformate is very dangerous when exposed to heat sources, sparks, flame, or oxidizers. Methyl chloroformate will react with water or steam to produce toxic and corrosive fumes. Vapors may travel to a source of ignition and flash back. Withdraw immediately in case of rising sound from venting safety device or any discoloration of tank due to fire. Toxic fumes of phosgene are produced when the material is heated to decomposition. Heat or steam should be avoided.Химическая реактивность
Reactivity with Water: Reacts slowly, evolving hydrogen chloride (hydrochloric acid). Reaction can be hazardous if water is hot; Reactivity with Common Materials: Corrodes rubber; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Flush with water, rinse with sodium bicarbonate or lime solution; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Профиль безопасности
Poison by ingestion, inhalation, and intraperitoneal routes. Moderately toxic by skin contact. Human systemic effects by inhalation: conjunctiva irritation and respiratory effects. Corrosive to skin, eyes, and mucous membranes. Very dangerous fire hazard when exposed to heat sources, sparks, flame, or oxidzers. Reacts with water or steam to produce toxic and corrosive fumes. When heated to decomposition it emits toxic fumes of Cl-, methyl chloroformate, and phosgene.Возможный контакт
Used in synthesis of pharmaceuticals; herbicides, plastics and other organic chemicals; as a solvent in the photographic industry; as a chemical intermediate in the production of other chemicals. In WWI it was used as military tear-producing warfare agent.Перевозки
UN1238 Methyl chloroformate, Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard, 3-Flammable liquid, 8-Corrosive material Inhalation Hazard Zone AНесовместимости
May form explosive mixture with air. Violent reaction with alkali metals; ethers. Incompatible with strong acids; strong bases; alcohols, oxidizers, dimethylsulfoxide; dimethyl formamide. Contact with water or moisture produces corrosive and poisonous hydrogen chloride gas, methanol and carbon monoxide. Corrodes metals in the presence of moisture. Attacks some plastics, rubber and coatings.Утилизация отходов
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal