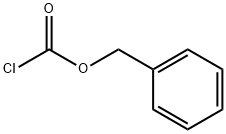
Бензил хлорформиа
- английское имяBenzyl chloroformate
- CAS №501-53-1
- CBNumberCB7853678
- ФормулаC8H7ClO2
- мольный вес170.59
- EINECS207-925-0
- номер MDLMFCD00000640
- файл Mol501-53-1.mol
| Температура плавления | -20 °C |
| Температура кипения | 103 °C20 mm Hg(lit.) |
| плотность | 1.212 g/mL at 20 °C |
| плотность пара | 1 (vs air) |
| давление пара | 1.39 psi ( 20 °C) |
| показатель преломления | n |
| Fp | 197 °F |
| температура хранения | Store at +2°C to +8°C. |
| растворимость | Miscible with ether, acetone and benzene. |
| форма | Solution |
| цвет | slightly yellow to slightly pink |
| Запах | Irritating; sharp, penetrating. |
| Растворимость в воде | decomposes |
| Чувствительный | Moisture Sensitive |
| Мерк | 14,1801 |
| Пределы воздействия | ACGIH: TWA 1 ppm OSHA: TWA 1 ppm(5 mg/m3) NIOSH: IDLH 10 ppm; Ceiling 1 ppm(5 mg/m3) |
| Стабильность | Stable. Incompatible with water, oxidizing agents. Combustible. |
| Справочник по базе данных CAS | 501-53-1(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 170BP0DD31 |
| Справочник по химии NIST | Benzyl chloroformate(501-53-1) |
| Система регистрации веществ EPA | Carbonochloridic acid, phenylmethyl ester (501-53-1) |
| UNSPSC Code | 12352108 |
| NACRES | NA.22 |
| Коды опасности | T,C,N,F | |||||||||
| Заявления о рисках | 45-20-34-48/22-50/53-67-65-63-48/20-11-43-37-23 | |||||||||
| Заявления о безопасности | 53-26-36/37/39-45-60-61-62-46-36/37 | |||||||||
| РИДАДР | UN 2924 3/PG 2 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | LQ5860000 | |||||||||
| F | 19 | |||||||||
| Температура самовоспламенения | 590 °C | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2915 90 70 | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | I | |||||||||
| Банк данных об опасных веществах | 501-53-1(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 3000 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)




-
сигнальный язык
опасность
-
вредная бумага
H335:Может вызывать раздражение верхних дыхательных путей.
H350:Может вызывать раковые заболевания.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P310:Немедленно обратиться за медицинской помощью.
Бензил хлорформиа химические свойства, назначение, производство
Описание
Benzyl chloroformate, also known as benzyl chlorocarbonate or Z-chloride, is the benzyl ester of chloroformic acid. It can be also described as the chloride of the benzyloxycarbonyl (Cbz or Z) group. In its pure form it is a water-sensitive oily colorless liquid, although impure samples usually appear yellow. It possesses a characteristic pungent odor and degrades in contact with water.The compound was first prepared by Leonidas Zervas in the early 1930s who used it for the introduction of the benzyloxycarbonyl protecting group, which became the basis of the Bergmann-Zervas carboxybenzyl method of peptide synthesis he developed with Max Bergmann. This was the first successful method of controlled peptide chemical synthesis and for twenty years it was the dominant procedure used worldwide until the 1950s. To this day, benzyl chloroformate is often used for amine group protection.
Химические свойства
colorless or light yellow oily liquid with rancid odor. soluble in ether, acetone, benzene and other organic solvents. It is used to protect amino groups in peptide synthesis.Использование
Benzyl chloroformate is widely used as a reactive chemical intermediate in plastic, pharmaceutical, agricultural and organic chemicals. It is useful for the introduction of carboxybenzyl (cbz) protecting group for amines such as aniline in organic synthesis. It is also involved in the synthesis of 1,2,4-oxadiazoles.Подготовка
Benzyl chloroformate is prepared in the lab by treating benzyl alcohol with phosgene:PhCH2OH + COCl2→ PhCH2OC(O)Cl + HCl
Phosgene is used in excess to minimise the production of the carbonate (PhCH2O)2C=O.
The use of phosgene gas in the lab preparation carries a very large health hazard, and has been implicated in the chronic pulmonary disease of pioneers in the usage of the compound such as Zervas.
прикладной
Benzyl chloroformate is used as a reagent in peptide synthesis to protect the amine functionality as the benzyloxycarbonyl (Cbz or Z) derivative.Cbz-protected anilines were prepared directly from aromatic carboxylic acids, sodium azide and Cbz-Cl.
Protecting reagent in peptide synthesis.
Общее описание
Benzyl chloroformate appears as a colorless liquid with an acrid odor. Vapors irritate eyes and mucous membranes. Corrosive to metals and tissue. Long-term inhalation of low concentrations or short-term inhalation of high concentrations can result in adverse health effects.Реакции воздуха и воды
Decomposes in moist air. Decomposes slowly in water to give corrosive hydrochloric acid and organic acids.Профиль реактивности
Benzyl chloroformate decomposes slowly in water forming benzyl alcohol, HCl, and CO2. Gives off HCl fumes in moist air. Reacts with bases, both organic and inorganic. Attacks many metals especially in humid atmosphere [Handling Chemicals Safely 1980. p. 476]. Catalytic impurity incidents involving the iron catalyzed decomposition of benzoyl chloroformate have caused several explosions. The iron presumably comes from corrosion of steel storage tanks [Loss Prev. Bull., 1975, (003), 2]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].Опасность
Highly toxic, emits very toxic phosgene fumes at 100C. Irritant to eyes.Угроза здоровью
Inhalation causes mucous membrane irritation. Eyes are irritated by excessive exposure to vapor. Liquid causes severe irritation of eyes and irritates skin. Ingestion causes irritation of mouth and stomach.Профиль безопасности
Poison by ingestion andinhalation routes. A powerful corrosive irritant. Thermallyunstable. Will react with water or steam to produce toxic and corrosive fumes and heat. Iron salts catalyze theexplosive decomposition of the ester. When heated todecomp.Методы очистки
The commercial material is usually better than 95% pure and may contain some toluene, benzyl alcohol, benzyl chloride and HCl. After long storage (e.g. two years at 4o, Greenstein and Winitz [The Chemistry of the Amino Acids Vol 2 p. 890, J Wiley and Sons NY, 1961] recommended that the liquid should be flushed with a stream of dry air, filtered and stored over sodium sulfate to remove CO2 and HCl which are formed by decomposition. It may further be distilled from an oil bath at a temperature below 85o because Thiel and Dent [Annalen 301 257 1898] stated that benzyloxycarbonyl chloride decarboxylates to benzyl chloride slowly at 100o and vigorously at 155o. Redistillation at higher vacuum below 85o yields material which shows no other peaks than those of benzyloxycarbonyl chloride by NMR spectroscopy. [Beilstein 6 IV 2278.] LACHRYMATORY and TOXIC.Бензил хлорформиа запасные части и сырье
сырьё
запасной предмет
- 2-(Boc-аминометил)-пиперидин
- Бензил 4-(бромметил)тетрагидро-1(2H)-пиридинкарбоксила
- Азтреонам
- 1-METHYL-[4,4']BIPIPERIDINYL
- Бета-аланин-трет-бутилэфир гидрохлорид
- Кларитромицин
- Lisinopril Dihydrate
- N-Карбобензилоксиглицин
- 4 - (Boc-аминометил) пиперидин
- ГЛИ-7-АМИНО-4-МЕТИЛКУМАРИН
- 1-Метилпиперазин-2-он
- Бензил карбама
- CARUMONAM
- 1H-азепин-3-карбоновая кислота, гексагидро-, метиловый эфир (9CI)
- Каптоприл
- (R) - (+)-1-Вос-3-аминопирролидин
- Procarbazine hydrochloride
- 2-PIPERAZIN-1-YL-ACETAMIDEHYDROCHLORIDE
- (R)-4-Boc-Piperazine-3-carboxylic acid
- N-1-BOC-N-4-CBZ-2-ПИПЕРАЗИНКАРБОНОВАЯ КИСЛОТА Т-БУТИЛОВЫЙ ЭФИР
1of6
Бензил хлорформиа поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +undefined-21-51877795 | China | 32965 | 60 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +8619521488211 | China | 2558 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 |
Бензил хлорформиа Обзор)
1of4