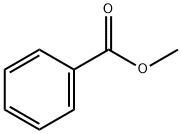
МЕТИЛБЕНЗОАТ
- английское имяMethyl benzoate
- CAS №93-58-3
- CBNumberCB8252119
- ФормулаC8H8O2
- мольный вес136.15
- EINECS202-259-7
- номер MDLMFCD00008421
- файл Mol93-58-3.mol
химическое свойство
| Температура плавления | -12 °C (lit.) |
| Температура кипения | 198-199 °C (lit.) |
| плотность | 1.088 g/mL at 20 °C (lit.) |
| плотность пара | 4.68 (vs air) |
| давление пара | <1 mm Hg ( 20 °C) |
| показатель преломления | n |
| FEMA | 2683 | METHYL BENZOATE |
| Fp | 181 °F |
| температура хранения | Store at +5°C to +30°C. |
| растворимость | ethanol: soluble60%, clear (1mL/4ml) |
| форма | Liquid |
| цвет | Clear colorless to pale yellow |
| Удельный вес | 1.087~1.095 (20℃) |
| Запах | at 1.00 % in dipropylene glycol. phenolic wintergreen almond floral cananga |
| Odor Type | phenolic |
| Биологические источники | synthetic |
| Пределы взрываемости | 8.6-20%(V) |
| Растворимость в воде | <0.1 g/100 mL at 22.5 ºC |
| Мерк | 14,6024 |
| Номер JECFA | 851 |
| БРН | 1072099 |
| Диэлектрическая постоянная | 6.6(20℃) |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents, strong acids, strong bases. |
| LogP | 2.12-2.2 at 20℃ |
| Вещества, добавляемые в пищу (ранее EAFUS) | METHYL BENZOATE |
| FDA 21 CFR | 172.515 |
| Справочник по базе данных CAS | 93-58-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 6618K1VJ9T |
| Справочник по химии NIST | Benzoic acid, methyl ester(93-58-3) |
| Система регистрации веществ EPA | Methyl benzoate (93-58-3) |
| UNSPSC Code | 85151701 |
| NACRES | NA.24 |
больше
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 22 | |||||||||
| Заявления о безопасности | 36 | |||||||||
| РИДАДР | UN 2938 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | DH3850000 | |||||||||
| Температура самовоспламенения | 510 °C | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29163100 | |||||||||
| Банк данных об опасных веществах | 93-58-3(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 3.43 g/kg (Smyth) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
МЕТИЛБЕНЗОАТ химические свойства, назначение, производство
Описание
Methyl benzoate is an organic compound. It is an ester with the chemical formula C6H5CO2CH3. It is a colorless liquid that is poorly soluble in water, but miscible with organic solvents. Methyl benzoate has a pleasant smell, strongly reminiscent of the fruit of the feijoa tree, and it is used in perfumery. It also finds use as a solvent and as a pesticide used to attract insects such as orchid bees.Химические свойства
Methyl benzoate is a colorless, oily, transparent liquid with a pleasant, fruity odor similar to ylang-ylang. It is soluble in methanol and ether, insoluble in water and glycerin, and miscible with ether. It is used in perfume bases such as ylang-ylang and tuberose types.Вхождение
Methyl benzoate can be isolated from the freshwater fern Salvinia molesta. It is one of many compounds that is attractive to males of various species of orchid bees, which apparently gather the chemical to synthesize pheromones; it is commonly used as bait to attract and collect these bees for study.Cocaine hydrochloride hydrolyzes in moist air to give methyl benzoate; drug - sniffing dogs are thus trained to detect the smell of methyl benzoate.
Подготовка
Methyl benzoate is manufactured by heating benzoic acid and dimethyl sulfate to high temperature, or by exchange between ethyl benzoate and methanol in KOH solution. It may also be produced by the alcoholysis of benzonitrile. It is a by-product of ozonolysis of water.прикладной
Methyl benzoate is a volatile aromatic ester compound widely used in perfumery industries. It is naturally occurring in guava, mango, and kiwifruit. It is one of many compounds that is attractive to males of various species of orchid bees, who apparently gather the chemical to synthesize pheromones. It can also be utilized as a precursor for:Selective synthesis of benzaldehyde using supported manganese oxide catalysts.
Preparation of benzophenone derivatives by reacting with aryl compounds via Friedel-Crafts acylation.
Определение
ChEBI: Methyl benzoate is a benzoate ester obtained by condensation of benzoic acid and methanol. It has a role as a metabolite and an insect attractant. It is a benzoate ester and a methyl ester.Общее описание
A crystalline solid or a solid dissolved in a liquid. Denser than water. Contact may slightly irritate skin, eyes and mucous membranes. May be slightly toxic by ingestion. Used to make other chemicals.Реакции воздуха и воды
Slightly soluble in water. Hydrolyzes slowly in contact with water .Профиль реактивности
Methyl benzoate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Methyl benzoate reacts with strong oxidizing agents and strong bases and hydrolyzes slowly in contact with water. .Угроза здоровью
Methyl benzoate is a mild skin irritant. Irritating to the eyes, nose, throat, upper respiratory tract, and skin. May cause allegic skin and respiratory reactions.Theacute oral toxicity in test animals was oflow order. The toxic symptoms in animalsfrom oral administration of this compoundwere tremor, excitement, and somnolence.The LD50 value varies with species. The oralLD50 values in mice and rats are 3330 and1350 mg/kg, respectively.Профиль безопасности
Moderately toxic by ingestion. Mildly toxic by skin contact. A skin and eye irritant. Combustible liquid when exposed to heat or flame; can react with oxidizing materials. To fight fire, use foam, CO2, dry chemical, water to blanket fire. When heated to decomposition it emits acrid smoke and irritating fumes.Возможный контакт
Used as food additive and as a solvent for cellulose esters and ethers, resins and rubber.Методы очистки
Wash the ester with dilute aqueous NaHCO3, then water, dry with Na2SO4 and fractionally distil it in a vacuum. [Beilstein 9 IV 283.]Утилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.МЕТИЛБЕНЗОАТ запасные части и сырье
сырьё
1of2
запасной предмет
1of3
МЕТИЛБЕНЗОАТ поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8617320528784 | China | 41 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +86-0552-4929304 +86-18055277008 |
China | 57 | 55 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | ||
| +86-13734021967 +8613734021967 |
China | 1001 | 58 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 |
МЕТИЛБЕНЗОАТ Обзор)
1of4