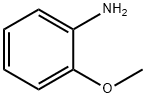
о-Анизидин
- английское имяo-Anisidine
- CAS №90-04-0
- CBNumberCB1286995
- ФормулаC7H9NO
- мольный вес123.15
- EINECS201-963-1
- номер MDLMFCD00007688
- файл Mol90-04-0.mol
| Температура плавления | 3-6 °C(lit.) |
| Температура кипения | 225 °C(lit.) |
| плотность | 1.092 g/mL at 25 °C(lit.) |
| давление пара | <0.1 at 25 °C (NIOSH, 1994) |
| показатель преломления | n |
| Fp | 210 °F |
| температура хранения | Refrigerator |
| растворимость | 14g/l |
| форма | Powder/Solid |
| пка | 4.52(at 25℃) |
| цвет | Off-white |
| РН | 7.3 (1g/l, H2O, 20℃) |
| Растворимость в воде | 13 g/L (20 ºC) |
| Чувствительный | Light Sensitive |
| Мерк | 14,667 |
| БРН | 386210 |
| констант закона Генри | 1.25 at 25 °C (approximate - calculated from water solubility and vapor pressure) |
| Пределы воздействия | Potential occupational carcinogen. NIOSH REL: TWA 0.5 mg/m3, IDLH 50 mg/m3; OSHA PEL: TWA 0.5 mg/m3; ACGIH TLV: TWA 0.1 ppm (adopted). |
| Диэлектрическая постоянная | 5.2300000000000004 |
| Стабильность | Stable. Incompatible with strong oxidizing agents, acid anhydrides, chloroformates, acids, some plastics, rubber. Air sensitive. |
| ИнЧИКей | VMPITZXILSNTON-UHFFFAOYSA-N |
| LogP | 1.180 |
| Справочник по базе данных CAS | 90-04-0(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 5 |
| FDA UNII | NUX042F201 |
| Предложение 65 Список | o-Anisidine |
| МАИР | 2A (Vol. Sup 7, 73, 127) |
| Справочник по химии NIST | Benzenamine, 2-methoxy-(90-04-0) |
| Система регистрации веществ EPA | o-Anisidine (90-04-0) |
| UNSPSC Code | 12352100 |
| NACRES | NA.22 |
| Коды опасности | T,Xi | |||||||||
| Заявления о рисках | 45-23/24/25-68 | |||||||||
| Заявления о безопасности | 53-45 | |||||||||
| РИДАДР | UN 2431 6.1/PG 3 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 0.5 mg/m3 [skin] | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | BZ5410000 | |||||||||
| F | 8 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29222200 | |||||||||
| Банк данных об опасных веществах | 90-04-0(Hazardous Substances Data) | |||||||||
| Токсичность | Acute oral LD50 for rats 2,000 mg/kg, wild birds 422 mg/kg, mice 1,400 mg/kg, rabbits 870 mg/kg (quoted, RTECS, 1985). | |||||||||
| ИДЛА | 50 mg/m3 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H301+H311+H331:Токсично при проглатывании, при контакте с кожей или при вдыхании.
H341:Предполагается, что данное вещество вызывает генетические дефекты.
H350:Может вызывать раковые заболевания.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P310+P330:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
P304+P340+P311:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью.
о-Анизидин химические свойства, назначение, производство
Описание
o-Anisidine was produced commercially in the United States from the 1920s until late 1950s. By 2009, worldwide only six industries manufactured o-anisidine, but none produced hydrochloride salt. o-Anisidine was available from 44 suppliers, including 20 US suppliers, and the hydrochloride salt was available from eight suppliers, including five US suppliers. US imports of o-anisidine and its hydrochloride salt are reported in the category ‘o-anisidines, p-anisidines, and p-phenetidine,’ and US exports are reported in the category ‘anisidines, dianisidines, phenetidines, and their salts.’ From 1989 to 2008, imports ranged from a high of over 4.6 million kg in 1996 to zero in 2007 and 2008, and exports ranged from zero to 262 000 kg. Reports filed under the US Environmental Protection Agency’s (EPA) Toxic Substances Control Act Inventory Up Rule indicated that United States production plus imports of o-anisidine totaled 500 000 lb–1 million lb in 1986, 1990, and 2006; 1 million–10 million lb in 1990 and 1998; and 10 000–500 000 lb in 2002.Химические свойства
Anisidine exists as ortho-, meta-, and paraisomers. They have characteristic amine (fishy) odors.o-Anisidine (or o-methoxyaniline) is an aromatic amine with a methoxyl group ortho to the amino group of aniline. It is a colorless to yellowish, pink, or reddish liquid.o-Anisidine is soluble in water and mineral oils, and miscible with alcohol, benzene, acetone, and diethylether. o-Anisidine hydrochloride, a salt of o-anisidine, is a grayish crystalline solid or powder at room temperature and is soluble in water (NTP, 2011).
Физические свойства
Colorless, yellow to reddish liquid with an amine-like odor. Becomes brown on exposure to air.Использование
Similar to other aromatic amines, o-anisidine may cause methemglobinemia and cancer in humans. It is used mainly as an intermediate for the production of azo dyes and pigments. Other industrial uses of o-anisidine include synthesis of other dyes and pharmaceuticals, as a corrosion inhibitor for steel, and as an antioxidant for polymercaptan resins (IARC, 1999; HSDB, 2012).Intermediate for azo dyes and for guaiacol.
Общее описание
Clear, yellowish to reddish or brown liquid with an amine (fishy) odor.Реакции воздуха и воды
o-Anisidine darkens on exposure to air. Insoluble in water.Профиль реактивности
o-Anisidine is sensitive to heat. o-Anisidine is also sensitive to exposure to light. o-Anisidine is incompatible with strong oxidizers. o-Anisidine is also incompatible with acids, acid chlorides, acid anhydrides and chloroformates. o-Anisidine will attack some forms of plastics, rubber and coatings. .Опасность
Strong irritant. Toxic when absorbed through the skin. Possible carcinogen.Угроза здоровью
o-Anisidine was carcinogenic in experimental animals.Пожароопасность
o-Anisidine is combustible.Профиль безопасности
Confirmed carcinogen. Moderately toxic by ingestion. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.Возможный контакт
Anisidines are used in the manufacture of azo dyes; pharmaceuticals; textile-processing chemicals Incompatibilities: Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine,bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Attacks some coatings and some forms of plastic and rubber.Канцерогенность
o-Anisidine is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity in experimental animals.Экологическая судьба
Biological. o-Anisidine should be biodegradable according to OECD guidelines (Brown and Labouerer (1983). Chemical/Physical. At influent concentrations (pH 3.0) of 10, 1.0, 0.1, and 0.01 mg/L, the GAC adsorption capacities were 52, 20, 7.8, and 3.0 mg/g, respectively. At pHs 7 and 9, the GAC adsorption capacities were 110, 50, 23, and 10 mg/g at influent concentrations of 10, 1.0, 0.1, and 0.01 mg/L, respectively (Dobbs and Cohen, 1980).Перевозки
UN2431 Anisidines, Hazard Class: 6.1; Labels: 6.1-Poisonous materialsМетоды очистки
It is separated from the m-and p-isomers by steam distillation. It is also separated from its usual synthetic precursor o-nitroanisole by dissolving it in dilute HCl (pH <2.0) extracting the nitro impurity with Et2O, adjusting the pH to ~8.0 with NaOH, extracting the amine into Et2O or steam distilling. Extract the distillate with Et2O, dry the extract (Na2SO4), filter, evaporate and fractionate the residual oil. Protect the almost colourless oil from light which turns it yellow in color. [Biggs & Robinson J Chem Soc 3881961, Nodzu et al. Yakugaku Zasshi (J Pharm Soc Japan) 71 713, 715 1951, Beilstein 13 IV 806.]Утилизация отходов
Dissolve in combustible solvent (alcohols, benzene, etc.) and spray solution into furnace equipped with afterburner and scrubber, or burn spill residue on sand and soda ash absorbent in a furnace.о-Анизидин запасные части и сырье
сырьё
1of2
запасной предмет
- 2-метоксибензонитрил
- Нафтол AS-BI фосфат
- Benzoic acid, 2-[[1-amino-7-[[4-[[6-amino-5-[(3-carboxy-4-hydroxyphenyl)azo]-1-hydroxy-3-sulfo-2-naphthalenyl]azo]-2-methoxyphenyl]azo]-8-hydroxy-3,6-disulfo-2-naphthalenyl]azo]-5-[[6-amino-5-[(3-carboxy-4-hydroxyphenyl)azo]-1-hydroxy-3-sul
- МЕТОКСАДИАЗОН
- N- (2-метоксифенил) ацетамид
- 3,8-DIMETHYL-1,10-PHENANTHROLINE
- 2-метокси-4-нитроанилина
- Naphthol AS-OL
- 4-AMINO-3-METHOXYPHENYLBORONIC ACID, PINACOL ESTER
- 1- (2-метоксифенил) пиперазина гидробромид
- N-(2-METHOXY-4-NITROPHENYL)ACETAMIDE
- 2-метоксифенилизоциана
- FAST RED SALT ITR
- Reactive Yellow KE-4RN
- 2-Метоксифенол
- 2-фторанизола
- 3,4-DIHYDRO-8-HYDROXY-4-OXOQUINOLINE-3-CARBOXYLIC ACID
- FAST RED B SALT
- Fast Scarlet RC Base
- SIRIUS YELLOW GC
- 2-метоксибензолтиол
- Disperse Orange 29
- 2'-Methylacetoacetanilide
- 5-Хлор-2-метоксианилин
- N-(4-AMINO-2-METHOXYPHENYL)ACETAMIDE
1of7
о-Анизидин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +8615387054039 | China | 427 | 58 | |
| +86-371-66670886 | China | 19902 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-021-57951555 +8617317452075 |
China | 1803 | 55 | |
| +undefined-21-51877795 | China | 32965 | 60 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 |