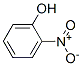
Нитрофенол
- английское имяNitrophenol
- CAS №25154-55-6
- CBNumberCB3875160
- ФормулаC6H5NO3
- мольный вес139.1088
- файл Mol25154-55-6.mol
химическое свойство
| Температура плавления | 44 °C |
| Температура кипения | 194 °C |
| плотность | 1.5 g/cm3 |
| Рейтинг продуктов питания EWG | 2 |
| FDA UNII | UV7XKM40LO |
| Система регистрации веществ EPA | Nitrophenol (25154-55-6) |
| РИДАДР | 1663 |
| Класс опасности | 6.1(b) |
| Группа упаковки | III |
| Банк данных об опасных веществах | 25154-55-6(Hazardous Substances Data) |
Нитрофенол химические свойства, назначение, производство
Химические свойства
There are three isomers of nitrophenol. The isomer of greatest concern, and the subject of ATSDR Toxicology Profile, is the p-isomer (4-nitrophenol). The meta-form is produced from m-nitroaniline, and the o-and p-isomers are produced by nitration of phenol. They are colorless to slightly yellowish crystals with an aromatic to sweetish odor.Общее описание
A yellow crystalline solid consisting of a mixture of chemically similar isomers. Slightly soluble in water. The primary hazard is the threat posed to the environment. Immediate steps should be taken to limit its spread to the environment. Nitrophenol is combustible though Nitrophenol may take some effort to ignite. Burns to give toxic oxides of nitrogen. Used in the manufacture of other chemicals.Реакции воздуха и воды
Slightly soluble in water.Профиль реактивности
Organonitrate compounds, such as Nitrophenol, range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. The aromatic nitro compounds may explode in the presence of a base such as sodium hydroxide or potassium hydroxide even in the presence of water or organic solvents. The explosive tendencies of aromatic nitro compounds are increased by the presence of multiple nitro groups. Phenols do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases.Угроза здоровью
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.Пожароопасность
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.Возможный контакт
(p-isomer): Possible risk of forming tumors, Suspected reprotoxic hazard. Nitrophenols are used as intermediates in production of dyes; photochemicals, pesticides, and pharmaceuticals; in leather tanning.Перевозки
UN1663 Nitrophenols (o-; m-; p-), Hazard Class: 6.1; Labels: 6.1-Poisonous materials.Несовместимости
Nitrophenols are strong oxidizers. Reacts violently with combustible and reducing agents. Contact with potassium hydroxide forms an explosive mixture. May explode on heating.Утилизация отходов
Controlled incineration-care must be taken to maintain complete combustion at all times. Incineration of large quantities may require scrubbers to control the emission of nitrogen oxides. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.