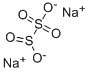
Метабисульфит натрия
- английское имяSodium metabisulfite
- CAS №7681-57-4
- CBNumberCB9854280
- ФормулаH2O5S2.2Na
- мольный вес192.12
- EINECS231-673-0
- номер MDLMFCD00213787
- файл Mol7681-57-4.mol
| Температура плавления | >300 °C (lit.) |
| плотность | 1.48 |
| Плотность накопления | 1000-1200kg/m3 |
| показатель преломления | 1.474 |
| температура хранения | Store at +5°C to +30°C. |
| растворимость | 540 g/L (20°C) |
| форма | Powder/Solid |
| Удельный вес | 1.48 |
| цвет | White to slightly yellow |
| РН | 3.5-5 (50g/l, H2O, 20℃) |
| Запах | Sulphorous |
| Водородный показатель | 4.5 at 50 g/l at 20 °C |
| Растворимость в воде | 540 g/L (20 ºC) |
| Чувствительный | Air & Moisture Sensitive |
| Мерк | 14,8638 |
| Пределы воздействия | ACGIH: TWA 5 mg/m3 NIOSH: TWA 5 mg/m3 |
| Стабильность | Stable. Incompatible with strong oxidizing agents, strong acids. Contact with strong acids releases a poisonous gas. May be moisture and air sensitive. |
| ИнЧИКей | HRZFUMHJMZEROT-UHFFFAOYSA-L |
| Справочник по базе данных CAS | 7681-57-4(CAS DataBase Reference) |
| FDA 21 CFR | 182.3766; 582.3766; 173.310 |
| Вещества, добавляемые в пищу (ранее EAFUS) | SODIUM METABISULFITE |
| Специальный комитет по веществам GRAS | Sodium metabisulfite |
| Рейтинг продуктов питания EWG | 2-4 |
| FDA UNII | 4VON5FNS3C |
| Система регистрации веществ EPA | Sodium metabisulfite (7681-57-4) |
| UNSPSC Code | 85151701 |
| NACRES | NB.24 |
| Коды опасности | Xn,Xi | |||||||||
| Заявления о рисках | 22-31-41-36/37/38-52 | |||||||||
| Заявления о безопасности | 26-39-46-36-16 | |||||||||
| РИДАДР | 3260 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 5 mg/m3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | UX8225000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2832 10 00 | |||||||||
| Группа упаковки | III | |||||||||
| Банк данных об опасных веществах | 7681-57-4(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 1540 mg/kg LD50 dermal Rat > 2000 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H318:При попадании в глаза вызывает необратимые последствия.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Метабисульфит натрия химические свойства, назначение, производство
Химические свойства
Sodium metabisulfite is a white crystalline powder. Sulfur dioxide odor. It may be considered the anhydride of 2 molecules of sodium disulfite.Использование
sodium metabisulfite is an anti-oxidant and reducing agent.Определение
ChEBI: An inorganic sodium salt composed of sodium and disulfite ions in a 2:1 ratio.Методы производства
Sodium metabisulfite is prepared by saturating a solution of sodium hydroxide with sulfur dioxide and allowing crystallization to occur; hydrogen is passed through the solution to exclude air. Sodium metabisulfite may also be prepared by saturating a solution of sodium carbonate with sulfur dioxide and allowing crystallization to occur, or by thermally dehydrating sodium bisulfite.Общее описание
Sodium metabisulfite (MBS, Sodium disulfite) is an inorganic salt. It is the sodium salt of disulphurous (pyrosulfurous) acid. It is widely used in textile dyeing, photography and paper industry. It is also commonly added to various food products and wines as a preservative.Опасность
Toxic by inhalation. Upper respiratory tract irritant. Questionable carcinogen.Угроза здоровью
Sodium metabisulfite may cause bronchospasm, oculonasal symptoms, and urticaria in sulfite-sensitive individuals; irritation of mucous membranes may occur from inhalation of the dust.Фармацевтические приложения
Sodium metabisulfite is used as an antioxidant in oral, parenteral, and topical pharmaceutical formulations, at concentrations of 0.01–1.0% w/v, and at a concentration of approximately 27% w/v in intramuscular injection preparations. Primarily, sodium metabisulfite is used in acidic preparations; for alkaline preparations, sodium sulfite is usually preferred. Sodium metabisulfite also has some antimicrobial activity, which is greatest at acid pH, and may be used as a preservative in oral preparations such as syrups.In the food industry and in wine production, sodium metabisulfite is similarly used as an antioxidant, antimicrobial preservative, and antibrowning agent. However, at concentrations above about 550 ppm it imparts a noticeable flavor to preparations. Sodium metabisulfite usually contains small amounts of sodium sulfite and sodium sulfate.
Контактные аллергены
This agent is frequently used as a preservative in pharmaceutical products, in the bread-making industry as an antioxidant, and it can induce contact dermatitis. It can be used as a reducing agent in photography and caused dermatitis in a photographic technician, probably acting as an aggravating irritative factor. Sodium metabisulfite contains a certain amount of sodium sulfite and sodium sulfate.Профиль безопасности
An inhalation hazard. Poison by intravenous route. Moderately toxic by parenteral route. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of SOx and Na2O.Безопасность
Sodium metabisulfite is widely used as an antioxidant in oral, topical, and parenteral pharmaceutical formulations; it is also widely used in food products.Although it is extensively used in a variety of preparations, sodium metabisulfite and other sulfites have been associated with a number of severe to fatal adverse reactions. These are usually hypersensitivity-type reactions and include bronchospasm and anaphylaxis. Allergy to sulfite antioxidants is estimated to occur in 5–10% of asthmatics, although adverse reactions may also occur in nonasthmatics with no history of allergy.
Following oral ingestion, sodium metabisulfite is oxidized to sulfate and is excreted in urine. Ingestion may result in gastric irritation, owing to the liberation of sulfurous acid, while ingestion of large amounts of sodium metabisulfite can cause colic, diarrhea, circulatory disturbances, CNS depression, and death.
In Europe, the acceptable daily intake of sodium metabisulfite and other sulfites used in foodstuffs has been set at up to 3.5 mg/kg body-weight, calculated as sulfur dioxide (SO2). The WHO has similarly also set an acceptable daily intake of sodium metabisulfite, and other sulfites, at up to 7.0 mg/kg body-weight, calculated as sulfur dioxide (SO2).
LD50 (rat, IV): 0.12 g/kg
Возможный контакт
Sodium metabisulfite is used as an antioxidant in pharmaceutical preparations and as a preservative in foods. People with asthma have a greater chance of having an allergic reaction with this chemical. Individuals allergic to sodium bisulfite (a food preservative found in some wine, fresh shrimp; packaged foods; and restaurant salads and potatoes) may have a severe reaction when exposed to sodium metabisulfite.Канцерогенность
Sodium metabisulfite was genotoxic in mice in vivo as determined by chromosomal aberration, micronucleus, and sperm shape assays. It was not mutagenic in bacterial assays.хранилище
On exposure to air and moisture, sodium metabisulfite is slowly oxidized to sodium sulfate with disintegration of the crystals. Addition of strong acids to the solid liberates sulfur dioxide.In water, sodium metabisulfite is immediately converted to sodium (Na+) and bisulfite (HSO3-) ions. Aqueous sodium metabisulfite solutions also decompose in air, especially on heating. Solutions that are to be sterilized by autoclaving should be filled into containers in which the air has been replaced with an inert gas, such as nitrogen. The addition of dextrose to aqueous sodium metabisulfite solutions results in a decrease in the stability of the metabisulfite.
The bulk material should be stored in a well-closed container, protected from light, in a cool, dry place.
Перевозки
UN1759 Corrosive solids, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name required. UN2693 Bisulfites, inorganic, aqueous solutions, n.o.s., Hazard class: 8; Labels: 8-Corrosive material.Несовместимости
Sodium metabisulfite reacts with sympathomimetics and other drugs that are ortho- or para-hydroxybenzyl alcohol derivatives to form sulfonic acid derivatives possessing little or no pharmacological activity. The most important drugs subject to this inactivation are epinephrine (adrenaline) and its derivatives. In addition, sodium metabisulfite is incompatible with chloramphenicol owing to a more complex reaction; it also inactivates cisplatin in solution.It is incompatible with phenylmercuric acetate when autoclaved in eye drop preparations.
Sodium metabisulfite may react with the rubber caps of multidose vials, which should therefore be pretreated with sodium metabisulfite solution.
Регуляторный статус
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (epidural;inhalation; IM and IV injections; ophthalmic solutions; oral preparations; rectal, topical, and vaginal preparations). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.Метабисульфит натрия запасные части и сырье
сырьё
запасной предмет
- 6-HYDROXY-7-METHOXY-4-PHENYLCOUMARIN
- 4-bromo-1-(bromomethyl)-2-(4-chlorophenyl)-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile
- 2-(4-chlorophenyl)-1-methyl-5-(trifluoromethyl)-1H-pyrrole-3-carbonitrile
- (1S,2S,3R,5S)-(+)-2,3-пинанедиол
- Ацетазоламид
- 5-Бром-6-хлорникотиновой кислоты
- Амидопирин
- 2,4-ДИХЛОРО-3-МЕТИЛБЕНЗОЙНАЯ КИСЛОТА
- valanea tanning extract
- friction reducer SAF
- oil-proof, water-proof finishing agent
- 6,6'-Ureylene-bis(1-naphthol-3-sulfonic acid)
- Hydroxocobalamin Hydrochloride
- 3-амино-5-хлорпиридин
- 1-(3-AMINO-2-CHLORO-PYRIDIN-4-YL)-ETHANONE
- 4-хлор-3-(трифторметил)бензальдегид
- 1,3,3-триметил-2-methyleneindoline
- 2-propylisonicotinonitrile
- 2-хлорбензолсульфонамида
- Салициламид
- Фолиевая кислота
- 3-Амино-2 ,4,6-трииодбензойная кислота
- 3-Nitroisonicotinic acid
- SULFONATED ALIPHATIC POLYESTER
- 1-диазо-2-нафтол-4-сульфоновой кислоты
- 4-метокси-2-нитроанилин
- 2-(4-CHLOROPHENYL)-4-BROMO-1-METHYL-5-(TRIFLUOROMETHYL)-1H-PYRROLE-3-CARBONITRILE
- Tannin resin glue
- Penetrating agent
- S-Карбоксиметил-L-цистеин
1of8
Метабисульфит натрия поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0551-65418684 +8618949823763 |
China | 25356 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-66697723 +86-17703311139 |
China | 563 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-86-4001020630 +8619831957301 |
China | 1000 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | ||
| +16837774622 | China | 295 | 58 | ||
| +8615713292910 | China | 341 | 58 | ||
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 |