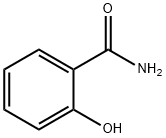
Салициламид
- английское имяSalicylamide
- CAS №65-45-2
- CBNumberCB4854078
- ФормулаC7H7NO2
- мольный вес137.14
- EINECS200-609-3
- номер MDLMFCD00007978
- файл Mol65-45-2.mol
| Температура плавления | 140-144 °C(lit.) |
| Температура кипения | 270°C |
| плотность | 1,175 g/cm3 |
| показатель преломления | 1.5323 (estimate) |
| Fp | 181°C/14mm |
| температура хранения | Inert atmosphere,Room Temperature |
| растворимость | methanol: 0.1 g/mL, clear |
| пка | pKa 8.13(H2O t = 37) (Uncertain) |
| форма | Crystalline Powder |
| цвет | White |
| Запах | Odorless |
| Водородный показатель | 5 (0.2% aq soln) |
| Растворимость в воде | <0.1 g/100 mL at 20 ºC |
| Разложение | 270°C |
| Мерк | 14,8328 |
| БРН | 742439 |
| Стабильность | Stable. Light sensitive. Incompatible with strong bases, strong oxidizing agents. |
| ИнЧИКей | SKZKKFZAGNVIMN-UHFFFAOYSA-N |
| LogP | 1.280 |
| FDA 21 CFR | 310.545 |
| Справочник по базе данных CAS | 65-45-2(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | EM8BM710ZC |
| Код УВД | N02BA05 |
| Справочник по химии NIST | Benzamide, 2-hydroxy-(65-45-2) |
| Система регистрации веществ EPA | o-Hydroxybenzamide (65-45-2) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 22-36/37/38-20/21/22 | |||||||||
| Заявления о безопасности | 26-36 | |||||||||
| РИДАДР | 3249 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | VN6475000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1(b) | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29214300 | |||||||||
| Банк данных об опасных веществах | 65-45-2(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in mice: 1.4 g/kg (Hart) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Салициламид химические свойства, назначение, производство
Описание
Salicylamide is less acidic (pKa 8.2) than other salicylic acid derivatives. Although poorly soluble in water, stable solutions can be formed at pH 9 through ionization of the phenolic group. It is absorbed from the GI tract on oral administration and is rapidly metabolized to inactive metabolites by intestinal mucosa, but not by hydrolysis. Activity appears to reside in the intact molecule. It is approximately 40 to 55% plasma protein bound, and it competes with other salicylates and acetaminophen for glucuronide conjugation, decreasing the extent of conjugation of these other drugs.Химические свойства
Salicylamide is a odorless white or slightly pink crystals. It is less acidic (pKa 8.2) than other salicylic acid derivatives. Although poorly soluble in water, stable solutions can be formed at pH 9 through ionization of the phenolic group.Использование
salicylamide is an analgesic, fungicide, and anti-inflammatory ingredient used to soothe the skin. It is an aromatic amide.Определение
ChEBI: The simplest member of the class of salicylamides derived from salicylic acid.Общее описание
Salicylamide, o-hydroxybenzamide, is a derivative of salicylicacid that is fairly stable to heat, light, and moisture. Itreportedly exerts a moderately quicker and deeper analgesiceffect than aspirin because of quicker CNS penetration. Its metabolismdiffers from aspirin, because it is not metabolized tosalicylic acid but rather excreted exclusively as the ether glucuronideor sulfate. Thus, as a result of lack of contributionfrom salicylic acid, it has a lower analgesic and antipyretic efficacythan that of aspirin. However, it can be used in place ofsalicylates for patients with a demonstrated sensitivity to salicylates.It is also excreted much more rapidly than other salicylates,which accounts for its lower toxicity. It is available inseveral nonprescription products, in combination with acetaminophenand phenyltoloxamine (e.g., Rid-A Pain compound,Cetazone T, Dolorex, Ed-Flex, Lobac) or with aspirin,acetaminophen, and caffeine (e.g., Saleto, BC Powder).Реакции воздуха и воды
Salicylamide darkens on exposure to air. . Insoluble in water.Профиль реактивности
Salicylamide is an amide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). Salicylamide may be sensitive to prolonged exposure to light.Пожароопасность
Flash point data for Salicylamide are not available; however, it is probably combustible.Клиническое использование
Whereas salicylamide is reported to be as effective as aspirin as an analgetic/antipyretic and is effective in relieving pain associated with arthritic conditions, it does not appear to possess useful anti-inflammatory activity. Thus, indications for the treatment of arthritic disease states are unwarranted, and its use is restricted to the relief of minor aches and pain at a dosage of 325 to 650 mg three or four times per day. Its effects in humans are not reliable, however, and its use is not widely recommended.Методы очистки
Crystallise the amide from water or repeatedly from CHCl3 [Nishiya et al. J Am Chem Soc 108 3880 1986]. [Beilstein 10 IV 169.] The anilide [87-17-2] M 213.2, m 135o crystallises from H2O. [Beilstein 12 H 500, 12 I 268, 12 II 256, 12 944.]Салициламид запасные части и сырье
Салициламид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +8617732866630 | China | 18147 | 58 | |
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | |
| +8617756083858 | China | 973 | 58 | |
| +86-18353166132 +86-18353166132 |
China | 983 | 58 | |
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +86-18633929156 +86-18633929156 |
China | 972 | 58 |