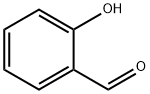
Салицилового альдегида
- английское имяSalicylaldehyde
- CAS №90-02-8
- CBNumberCB6127950
- ФормулаC7H6O2
- мольный вес122.12
- EINECS201-961-0
- номер MDLMFCD00003317
- файл Mol90-02-8.mol
| Температура плавления | 1-2 °C (lit.) |
| Температура кипения | 197 °C (lit.) |
| плотность | 1.146 g/mL at 25 °C (lit.) |
| плотность пара | 4.2 (vs air) |
| давление пара | 1 mm Hg ( 33 °C) |
| показатель преломления | n |
| FEMA | 3004 | SALICYLALDEHYDE |
| Fp | 170 °F |
| температура хранения | -20°C |
| растворимость | 4.9g/l |
| форма | Liquid |
| пка | 8.37(at 25℃) |
| цвет | Clear yellow |
| РН | 6-8 (H2O, 20℃)Not applicable |
| Запах | Bitter almonds |
| Odor Type | medicinal |
| Биологические источники | synthetic |
| Растворимость в воде | slightly soluble |
| Чувствительный | Air & Light Sensitive |
| Мерк | 14,8326 |
| Номер JECFA | 897 |
| БРН | 471388 |
| Диэлектрическая постоянная | 13.9(20℃) |
| Пределы воздействия | ACGIH: TWA 5 ppm (Skin) OSHA: TWA 5 ppm(19 mg/m3) NIOSH: IDLH 250 ppm; TWA 5 ppm(19 mg/m3); Ceiling 15.6 ppm(60 mg/m3) |
| Стабильность | Stable. Combustible. Incompatible with strong bases, strong reducing agents, strong acids, strong oxidizing agents. |
| ИнЧИКей | SMQUZDBALVYZAC-UHFFFAOYSA-N |
| LogP | 1.66 at 25℃ and pH6.2-6.3 |
| Вещества, добавляемые в пищу (ранее EAFUS) | SALICYLALDEHYDE |
| FDA 21 CFR | 172.515 |
| Справочник по базе данных CAS | 90-02-8(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 17K64GZH20 |
| Справочник по химии NIST | Benzaldehyde, 2-hydroxy-(90-02-8) |
| Система регистрации веществ EPA | Salicylaldehyde (90-02-8) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn,N | |||||||||
| Заявления о рисках | 21/22-36/38-68-36/37/38-20/21/22-51-36-51/53-22 | |||||||||
| Заявления о безопасности | 26-36/37-36/37/39-36-61-64-29/35 | |||||||||
| РИДАДР | 3082 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | VN5250000 | |||||||||
| F | 8-10-23 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1(b) | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 29122990 | |||||||||
| Банк данных об опасных веществах | 90-02-8(Hazardous Substances Data) | |||||||||
| Токсичность | MLD in rats (mg/kg): 900-1000 s.c. (Binet) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H302:Вредно при проглатывании.
H341:Предполагается, что данное вещество вызывает генетические дефекты.
H411:Токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P273:Избегать попадания в окружающую среду.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Салицилового альдегида химические свойства, назначение, производство
Описание
Salicylaldehyde (2-hydroxybenzaldehyde) is an organic compound with the formula C6H4CHO-2-OH. Along with 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde. It is a colorless or pale yellow liquid with a bitter almond odor and a burning taste. It is soluble in alcohol, benzene, and ether, and very slightly soluble in water. Salicylaldehyde is found in shrubs of the genus Spiraea and is usually produced from phenol by the action of chloroform in the presence of an alkali base. It is used in the production of coumarin, saligenin, and salicylaldoxime (an important analytical reagent), and also in analytical chemistry—for example, to detect hydrazine. Besides, salicylaldehyde is a key precursor to various chelating agents and a flavouring ingredient.Химические свойства
Salicylaldehyde is a colourless to yellow oily liquid with a pungent, irritating, bitter, almond-like odor similar to benzaldehyde, acetophenone and nitrobenzene, but with phenolic notes. It has a nut-like, coumarin flavor at low levels. slightly soluble in water, soluble in ethanol and ether. It turns purple in case of ferric chloride.Вхождение
Occurs frequently in nature; in the flowers of Spirea ulmaria and other Spireae, in the roots of Crepis foetida L., in the fruits of Pinus avium, in the rind of Rauqolfia caffra, in the leaves of Ceanothus velutinus and in the essential oil of Cinnamomum cassia and of tobacco leaves. Also reported found in grapes, tomato, baked potato, cinnamon bark, cassia leaf, peppermint oil, pennyroyal oil, parmesan cheese, butter, milk powder, roasted chicken, beer, rum, Japanese whiskey, sherry, coffee, tea, soybean, mushroom, buckwheat, Bourbon vanilla, Chinese quince, Muscat grape, vanilla and mastic gum oil.Подготовка
Salicylaldehyde is synthesized from phenol, chloroform, and alkali according to the Reimer–Tiemman method, which was developed in 1876. starting material for the manufacture of coumarin.Определение
ChEBI: Salicylaldehyde is a hydroxybenzaldehyde carrying a hydroxy substituent at position 2. It has a role as a nematicide and a plant metabolite.Общее описание
Liquid; colorless or pale yellow; bitter almond odor. Sinks and mixes slowly in water.Профиль реактивности
Salicylaldehyde is an aldehyde. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation.Угроза здоровью
Salicylaldehyde is a skin irritant; 500 mg/daycaused moderate irritation to rabbit skin. Itcan have injurious effects on fertility. Studieson rats indicate that subcutaneous administrationof salicylaldehyde in a high doseof >400 mg/kg can produce developmentalabnormalities, fetal death, and postimplantationmortality.The toxicity of this compound, however,is low. No toxic symptoms were noted.
LD50 value, oral (rats): 520 mg/kg
LD50 value, skin (rats): 600 mg/kg.
Пожароопасность
Combustible. Can react with oxidizing materials.Методы очистки
It is precipitated as the bisulfite addition compound by pouring the aldehyde slowly and with stirring into a 25% solution of NaHSO3 in 30% EtOH, then standing for 30minutes. The precipitate, after filtering at the pump, and washing with EtOH, is decomposed with aqueous 10% NaHCO3, and the aldehyde is extracted into diethyl ether, dried with Na2SO4 or MgSO4, and distilled, under reduced pressure. Alternatively, salicylaldehyde is precipitated as its Cu complex by adding it to warm, saturated aqueous Cu(OAc)2, shaking and standing in ice. The precipitate is filtered off, washed with EtOH, then Et2O, and decomposed with 10% H2SO4; the aldehyde is extracted into Et2O, dried and vacuum distilled. It was also purified by dry column chromatography on Kieselgel G [Nishiya et al. J Am Chem Soc 108 3880 1986]. The acetyl derivative has m 38-39o (from pet ether or EtOH) and b 142o/18mm, 253o/atm. [Beilstein 8 IV 176.] The oxime, [94-67-7] M 137.1, crystallises CHCl3/pet ether (b 40-60o) with m 57o [Beilstein 8 IV 203.]Утилизация отходов
Salicylaldehyde is burned in a chemicalincinerator equipped with an afterburner andscrubber.использованная литература
- https://en.wikipedia.org/wiki/Salicylaldehyde
- https://pubchem.ncbi.nlm.nih.gov/compound/salicylaldehyde#section=Top
- http://www.wisegeek.com/what-is-salicylaldehyde.htm
- https://www.merriam-webster.com/medical/salicylaldehyde
- http://encyclopedia2.thefreedictionary.com/Salicylaldehyde
Салицилового альдегида запасные части и сырье
сырьё
запасной предмет
- L(+)-альфа-фенилглицин
- 3-МЕТИЛ-ХРОМ-2-ОН
- Кумарин
- 5-(2-ETHOXYPHENYL)-1-METHYL-3-N-PROPYL-1,6-DIHYDRO-7H-PYRAZOLO[4,3-D]-7-PYRIMIDINONE
- a copper chelate
- 2-БЕНЗОФУРАНКАРБОНОВАЯ КИСЛОТА, ЭТИЛОВЫЙ ЭФИР
- Амиодарон
- 1,3,3-ТРИМЕТИЛИНДОЛИНОБЕНЗОПИРИЛОСПИРАН
- 5-хлорсалицилальдегида
- Бунитролол
1of6
Салицилового альдегида поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0519-85551759 +8613506123987 |
China | 8840 | 58 | ||
| +86-371-66670886 | China | 19902 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | ||
| +undefined-86-25-86655873 | China | 114 | 58 | ||
| +86-13734021967 +8613734021967 |
China | 1001 | 58 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 |