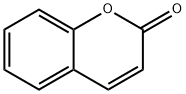
Кумарин
- английское имяCoumarin
- CAS №91-64-5
- CBNumberCB3112168
- ФормулаC9H6O2
- мольный вес146.14
- EINECS202-086-7
- номер MDLMFCD00006850
- файл Mol91-64-5.mol
| Температура плавления | 68-73 °C (lit.) |
| Температура кипения | 298 °C (lit.) |
| плотность | 0.935 |
| давление пара | 0.01 mm Hg ( 47 °C) |
| показатель преломления | 1.5100 (estimate) |
| Fp | 162 °C |
| температура хранения | Store below +30°C. |
| растворимость | 1.7g/l |
| форма | Crystals or Crystalline Powder |
| цвет | White |
| Водородный показатель | Non' uorescence (9.5) to light green ' uorescence (10.5) |
| Запах | at 10.00 % in dipropylene glycol. sweet hay tonka new mown hay |
| Odor Type | tonka |
| Биологические источники | synthetic |
| Растворимость в воде | 1.7 g/L (20 ºC) |
| λмакс | 275nm |
| Мерк | 14,2562 |
| БРН | 383644 |
| Основное приложение | color filter, organic electroluminescent devices, liquid crystal displays, field emission displays, inks, nickel plating, detergents, deodorant for shoes, petroleum products, cigarettes, personal care products, cosmetics, sunscreen cream, perfumes, nucleic acid sequencing, antiinflammatory agent, treatment of cancer, neurotransmission disorders, bleeding disorders, cerebrovascular disease, thrombosis, hemorrhoids, rheumatic disease, arthritic disease, epilepsy, vaginitis, painkiller, teeth whitening agent, skin whitening agent, wound healing promoter |
| ИнЧИКей | ZYGHJZDHTFUPRJ-UHFFFAOYSA-N |
| LogP | 1.39 at 25℃ |
| FDA 21 CFR | 189.130 |
| Вещества, добавляемые в пищу (ранее EAFUS) | COUMARIN--PROHIBITED |
| Справочник по базе данных CAS | 91-64-5(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 4-7 |
| Словарь онкологических терминов NCI | coumarin |
| FDA UNII | A4VZ22K1WT |
| Словарь наркотиков NCI | coumarin |
| Справочник по химии NIST | Coumarin(91-64-5) |
| МАИР | 3 (Vol. Sup 7, 77) 2000 |
| Система регистрации веществ EPA | Coumarin (91-64-5) |
| UNSPSC Code | 85151701 |
| NACRES | NA.25 |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 22-40-36/37/38-20/21/22-43 | |||||||||
| Заявления о безопасности | 36-36/37-26 | |||||||||
| РИДАДР | UN 2811 6.1/PG 3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | GN4200000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29322010 | |||||||||
| Банк данных об опасных веществах | 91-64-5(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats, guinea pigs: 680, 202 mg/kg (Jenner) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
H301+H311:Токсично при проглатывании или при контакте с кожей.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
Кумарин химические свойства, назначение, производство
Описание
Coumarin is a naturally occurring Benzopyrone compound. It is found in a large number of plants belonging to many different families including tonka beans, woodruff, lavender oil, cassia, melilot (sweet clover), and other plants. It is found in edible plants such as strawberries, cinnamon, peppermint, green tea, carrots, and celery, as well as in partially fermented tea, red wine, beer, and other foodstuffs. Concentrations range from 87 000 ppm in cassia and 40 000 ppm in cinnamon to 20 ppm in peppermint and 5 ppb in tangerines.Химические свойства
Coumarin occurs widely in nature and determines, for example, the odor of woodruff. It forms white crystals (mp 70.6°C) with a hay-like, spicy odor. When treated with dilute alkali, coumarin is hydrolyzed to the corresponding coumarinic acid salt [(Z)-2-hydroxycinnamic acid]. Heating with concentrated alkali or with sodium ethanolate in ethanol results in the formation of o-coumaric acid salts [(E)-2-hydroxycinnamic acid]. 3,4-Dihydrocoumarin is obtained by catalytic hydrogenation, for example, with Raney nickel as a catalyst; octahydrocoumarin is obtained if hydrogenation is carried out at high temperature (200–250°C).Вхождение
Found in many plants and essential oils such as cassia, melilot, orchid, lavender and balsam of Peru (Sp?th, 1937; Gildemeister & Hoffman, 1966).Использование
coumarin is considered a blood thinner, it can also increase blood flow. Some sources cite anti-oxidant capacities, as well. It is a specific plant constituent and is what creates the fragrance of freshly mowed hay. Coumarin is found in such plants as cherries, lavender, licorice, and sweet clover.Подготовка
Coumarin is currently produced by Perkin synthesis from salicylaldehyde. In the presence of sodium acetate, salicylaldehyde reacts with acetic anhydride to produce coumarin and acetic acid. The reaction is carried out in the liquid phase at elevated temperature.A process for the production of coumarin from hexahydrocoumarin by dehydrogenation has also been elaborated.
Since the odor of coumarin is relatively weak, strong-smelling by-products (e.g., vinylphenol) must be removed. Many purification methods have been reported and patented.
Определение
A colorless crystalline compound with a pleasant odor, used in making perfumes. On hydrolysis with sodium hydroxide it forms coumarinic acid.Общее описание
Colorless crystals, flakes or colorless to white powder with a pleasant fragrant vanilla odor and a bitter aromatic burning taste.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
Coumarin is sensitive to exposure to light. Coumarin is also sensitive to heat. Coumarin is incompatible with strong acids, strong bases and oxidizers. Coumarin is hydrolyzed by hot concentrated alkalis. Coumarin can be halogenated, nitrated and hydrogenated (in the presence of catalysts).Опасность
Toxic by ingestion; carcinogenic. Use in food products prohibited (FDA). Questionable carcinogen.Угроза здоровью
SYMPTOMS: Exposure to Coumarin may cause narcosis. It may also cause irritation and liver damage.Пожароопасность
Coumarin is combustible.Биологическая активность
Oral anticoagulants can be prepared from compounds with coumarin as a base. Coumarin has been known for well over a century and, in addition to its use pharmaceutically, it is also an excellent odor-enhancing agent. However, because of its toxicity, it is not permitted in food products in the United States (Food and Drug Administration). One commercial drug is 3-(alpha-acetonyl-4-nitrobenzyl)- 4-hydroxycoumarin. This drug reduces the concentration of prothrombin in the blood and increases the prothrombin time by inhibiting the formation of prothrombin in the liver. The drug also interferes with the production of factors VII, IX, and X, so that their concentration in the blood is lowered during therapy. The inhibition of prothrombin involves interference with the action of vitamin K, and it has been postulated that the drug competes with vitamin K for an enzyme essential for prothrombin synthesis. Another commercial drug is bis-hydroxy-coumarin, C19H12O6. The actions of this drug are similar to those just described.Контактные аллергены
Coumarin is an aromatic lactone naturally occurring in Tonka beans and other plants. As a fragrance allergen, it has to be mentioned by name in cosmetics within the EUПрофиль безопасности
Poison by ingestion, intraperitoneal, and subcutaneous routes. Questionable carcinogen with experimental tumorigenic data. Experimental teratogenic effects. Mutation data reported. Combustible when exposed to heat or flame. When heated to decomposition it emits acrid smoke and fumes. See also KETONES and ANHYDRIDES.Экологическая судьба
Coumarin toxicity is a function of blood and target tissue levels of coumarin relative to the metabolic capacity of the target organ. Cellular toxicity results when the formation of the toxic moieties exceeds the capacity of the cell to detoxify. This can have significant impact when comparing dosing by gavage to dietary exposure.Методы очистки
Coumarin crystallises from ethanol or water and sublimes in vacuo at 43o [Srinivasan & deLevie J Phys Chem 91 2904 1987]. [Beilstein 17/10 V 143.]Кумарин запасные части и сырье
Кумарин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-15531157085 +86-15531157085 |
China | 8808 | 58 | |
| +86-13131129325 | China | 5872 | 58 | |
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | |
| +8618629063126 | China | 449 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +86-0531-8875-2665 +8613153039501 |
China | 662 | 58 | |
| +86-(0)57185586718 +86-13336195806 |
China | 29730 | 60 | |
| +86-0371-55170693 +86-19937530512 |
China | 21630 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | |
| +86-13734021967 +8613734021967 |
China | 1001 | 58 |