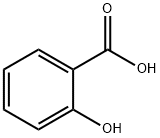
Салициловая кислота
- английское имяSalicylic acid
- CAS №69-72-7
- CBNumberCB1680010
- ФормулаC7H6O3
- мольный вес138.12
- EINECS200-712-3
- номер MDLMFCD00002439
- файл Mol69-72-7.mol
| Температура плавления | 158-161 °C(lit.) |
| Температура кипения | 211 °C(lit.) |
| Плотность накопления | 400-500kg/m3 |
| плотность | 1.44 |
| плотность пара | 4.8 (vs air) |
| давление пара | 1 mm Hg ( 114 °C) |
| FEMA | 3985 | 2-HYDROXYBENZOIC ACID |
| показатель преломления | 1,565 |
| Fp | 157 °C |
| температура хранения | 2-8°C |
| растворимость | ethanol: 1 M at 20 °C, clear, colorless |
| пка | 2.98(at 25℃) |
| форма | Solid |
| цвет | White to off-white |
| РН | 3.21(1 mM solution);2.57(10 mM solution);2.02(100 mM solution); |
| Запах | at 100.00 %. faint phenolic nutty |
| Водородный показатель | Non0 uorescence (2.5) to dark blue 0 uorescence (4.0) |
| Odor Type | nutty |
| Биологические источники | synthetic |
| Растворимость в воде | 1.8 g/L (20 ºC) |
| Чувствительный | Light Sensitive |
| λмакс | 210nm, 234nm, 303nm |
| Мерк | 14,8332 |
| Номер JECFA | 958 |
| Сублимация | 70 ºC |
| БРН | 774890 |
| Стабильность | Stable. Substances to be avoided include oxidizing agents, strong bases, iodine, fluorine. Combustible. Sensitive to light. |
| Основное приложение | Semiconductors, nanoparticles, photoresists, lubricating oils, UV absorbers, adhesive, leather, cleaner, hair dye, soaps, cosmetics, pain medication, analgesics, antibacterial agent, treatment of dandruff, hyperpigmented skin, tinea pedis, onychomycosis, osteoporosis, beriberi, fungicidal skin disease, autoimmune disease |
| ИнЧИКей | YGSDEFSMJLZEOE-UHFFFAOYSA-N |
| LogP | 2.01 |
| Вещества, добавляемые в пищу (ранее EAFUS) | SALICYLIC ACID |
| FDA 21 CFR | 175.105; 175.300; 177.2600; 310.545 |
| Справочник по базе данных CAS | 69-72-7(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 2-4 |
| FDA UNII | O414PZ4LPZ |
| Код УВД | D01AE12,S01BC08 |
| Справочник по химии NIST | Benzoic acid, 2-hydroxy-(69-72-7) |
| Система регистрации веществ EPA | Salicylic acid (69-72-7) |
| Информация о косметике | Salicylic Acid |
| UNSPSC Code | 41116107 |
| NACRES | NA.71 |
| Коды опасности | Xn,F | |||||||||
| Заявления о рисках | 22-41-36/37/38-36-20/21/22-11 | |||||||||
| Заявления о безопасности | 26-39-37/39-36-36/37-16 | |||||||||
| РИДАДР | UN 1648 3 / PGII | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | VO0525000 | |||||||||
| Температура самовоспламенения | 500 °C | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29182100 | |||||||||
| Банк данных об опасных веществах | 69-72-7(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 i.v. in mice: 500 mg/kg (Sota) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H318:При попадании в глаза вызывает необратимые последствия.
H361d:Предполагается, что данное вещество может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Салициловая кислота химические свойства, назначение, производство
Описание
Salicylic acid (from Latin salix, willow tree, from the bark of which the substance used to be obtained) is a mono hydroxy benzoic acid, a type of phenolic acid and a beta hydroxy acid. This colorless crystalline organic acid is widely used in organic synthesis and functions as a plant hormone. It is derived from the metabolism of salicin. In addition to being an important active metabolite of aspirin (acetylsalicylic acid), which acts in part as a prod rug to salicylic acid, it is probably best known for its use in anti-acne treatments. The salts and esters of salicylic acid are known as salicylates.Химические свойства
Salicylic acid has the formula C6H4(OH) COOH, where the OH group is ortho to the carboxyl group. It is also known as 2- hydroxybenzoic acid. It is poorly soluble in water (2 g / L at 20 °C). Aspirin (acetyl salicylic acid or ASA) can be prepared by the esterification of the phenolic hydroxyl group of salicylic acid with the acetyl group from acetic anhydride or acetyl chloride.Физические свойства
Salicylic acid. Appearance: white crystalline powder. Solubility: Absolutely soluble in ethanol, soluble in ether and chloroform, slightly soluble in water and anhydrous ether. Stability: Stable at room temperature, discomposes into phenol and carbon dioxide after rapidly heated. It’s partially acidic.Aspirin. Appearance: white crystal and decomposes at 136–140? °C. Melting point: 136?°C.?Aspirin is the acetyl derivative of salicylic acid with weak acidity. Its acidity coefficient is 3.5 at 25?°C. Stability: Aspirin decomposes rapidly in ammonium acetate, alkali metal of acetate, carbonate, citrate or hydroxide solutions. There are two crystal forms of aspirin including crystal form I and II.
Вхождение
Unripe fruits and vegetables are natural sources of salicylic acid, particularly blackberries, blueberries, cantaloupes, dates, raisins, kiwi fruits, guavas, apricots, green pepper, olives, tomatoes, radish and chicory; also mushrooms. Some herbs and spices contain quite high amounts, although meat, poultry, fish, eggs and dairy products all have little to no salicylates. Of the legumes, seeds, nuts, and cereals, only almonds, water chestnuts and peanuts have significant amounts.История
The Greek physician Hippocrates wrote in the 5th century BC about a bitter powder extracted from willow bark that could ease aches and pains and reduce fevers . This remedy was also mentioned in texts from ancient Sumer , Lebanon , and As syria .The active extract of the bark, called salicin, after the Latin name for the white willow (Salix alba), was isolated and named by the German chemist Johann Andreas Buchner in 1826. Raffaele Piria, an Italian chemist was able to convert the substance into a sugar and a second component, which on oxidation becomes salicylic acid.
Salicylic acid was also isolated from the herb meadowsweet (Filipendula ulmaria, formerly classified as Spiraea ulmaria) by German researchers in 1839. While their extract was somewhat effective, it also caused digestive problems such as gastric irritation, bleeding, diarrhea, and even death when consumed in high doses.
Использование
Salicylic Acid is an Impurity of Acetylsalicylic Acid (A187780). Acetylsalicylic acid Impurity B.Методы производства
Salicylic acid is biosynthesized from the amino acid phenylalanine. In Arabidopsis thaliana it can also be synthesized via a phenylalanine - independent pathway.Sodium salicylate is commercially prepared by treating sodium phenolate ( the sodium salt of phenol ) with carbon dioxide at high pressure (100 atm ) and high temperature (390K) -a method known as the Kolbe-Schmitt reaction. Acidification of the product with sulfuric acid gives salicylic acid :
It can also be prepared by the hydrolysis of aspirin (acetylsalicylic acid) or methyl salicylate (oil of winter green) with a strong acid or base.
Подготовка
Prepared by heating sodium phenolate with carbon dioxide under pressureОпределение
ChEBI: A monohydroxybenzoic acid that is benzoic acid with a hydroxy group at the ortho position. It is obtained from the bark of the white willow and wintergreen leaves.Показания
Salicylic acid is a β-hydroxy acid that penetrates into the sebaceous gland and has comedolytic and anti-inflammatory properties. It can be used as an adjunctive therapy and is found in cleansers, toners, masks, and peels.Salicylic acid is keratolytic and at concentrations between 3% and 6% causes softening of the horny layers and shedding of scales. It produces this desquamation by solubilizing the intercellular cement and enhances the shedding of corneocytes by decreasing cell-to-cell cohesion. In concentrations >6%, it can be destructive to tissue. Application of large amounts of the higher concentration of salicylic acid can also result in systemic toxicity. Salicylic acid is used in the treatment of superficial fungal infections, acne, psoriasis, seborrheic dermatitis, warts, and other scaling dermatoses. When it is combined with sulfur, some believe that a synergistic keratolytic effect is produced. Common preparations include a 3% and 6% ointment with equal concentration of sulfur; 6% propylene glycol solution (Keralyt); 5% to 20% with equal parts of lactic acid in flexible collodion for warts (Duofilm, Occlusal); in a cream base at any concentration for keratolytic effects; as a 60% ointment for plantar warts; and in a 40% plaster on velvet cloth for the treatment of calluses and warts (40% salicylic acid plaster).
Общее описание
Odorless white to light tan solid. Sinks and mixes slowly with water.Реакции воздуха и воды
Sublimes and forms vapor or dust that may explode [USCG, 1999].Опасность
Respiratory alkalosis, hyperkalemia, hyperthermia, dehydration, convulsions, shock, res- piratory paralysis, respiratory acidosis, lesions and death from respiratory collapse; fetotoxic.Угроза здоровью
Inhalation of dust irritates nose and throat. Vomiting may occur spontaneously if large amounts are swallowed. Contact with eyes causes irritation, marked pain, and corneal injury which should heal. Prolonged or repeated skin contact may cause marked irritation or even a mild burn.Механизм действия
Salicylic acid has been shown to work through several different pathways. It produces its anti - inflammatory effects via suppressing the activity of cyclo oxygenase (COX), an enzyme which is responsible for the production of pro - inflammatory mediators such as the prostaglandins. Notably, it does this not by direct inhibition of COX, unlike most other non-steroidal anti-inflammatory drugs (NSAIDs), but instead by suppression of the expression of the enzyme (via a yet-un elucidated mechanism) . Salicylic acid has also been shown to activate adenosine monophosphate-activated protein kinase (AMPK), and it is thought that this action may play a role in the anticancer effects of the compound and its prod rugs aspirin and salsalate. In addition, the anti diabetic effects of salicylic acid are likely mediated by AMPK activation primarily through allosteric conformational change that increases levels of phosphorylation. Salicylic acid also uncouples oxidative phosphorylation which leads to increased ADP:ATP and AMP:ATP ratios in the cell. Consequently, salicylic acid may alter AMPK activity and subsequently exert its anti-diabetic properties through altered energy status of the cell. Even in AMPK knock - out mice, however, there is an anti-diabetic effect demonstrating that there is at least one additional, yet - unidentified action of the compound.Фармаколо?гия
Aspirin is a nonsteroidal anti-inflammatory drug (NSAID). The main pharmacological effect is to inhibit prostaglandin metabolism and thromboxane synthesis by inhibiting prostaglandin metabolism-required cyclooxygenase, via irreversible acetylation of 530 serine residues in the hydroxyl of COX-1 polypeptide chain, which results in COX-1 inactivation, blocks the conversion of arachidonic acid into thromboxane A2 pathway and then inhibits the platelet aggregation.Prostaglandin is a hormone produced locally in the body. It can pass the pain to the brain, regulate body temperature in the hypothalamus and cause inflammation. Inhibition of prostaglandin synthesis can have antipyretic, analgesic, antiinflammatory and antirheumatic effects. The adverse effects of aspirin are mainly gastrointestinal symptoms such as nausea, vomiting, upper abdominal discomfort or pain. It can also cause allergic reactions, cardiotoxicity, liver and kidney damage and Wright’s syndrome. In addition, high doses of aspirin can cause salicylic acid reactions such as headache, dizziness, tinnitus, hearing loss and other central nervous system symptoms.
Клиническое использование
The clinical application of aspirin varies with the therapeutic dose. Low-dose aspirin (75–300?mg/day) has antiplatelet aggregation effect and can be used to prevent and treat the coronary and cerebrovascular thrombosis and other postoperative thrombosis. The middle dose of aspirin (0.5–3? g/day) has antipyretic analgesic effects, so it is commonly used in the treatment of fever, headache, toothache, neuralgia, muscle pain and menstrual pain. High doses of aspirin (more than 4?g/day) have anti-inflammatory and antirheumatic effects for the treatment of acute rheumatic fever and rheumatoid arthritis. In addition, aspirin is used for the treatment of skin and mucous membrane lymphadenopathy (Kawasaki disease) in paediatric.Побочные эффекты
Salicylic acid's side effects include erythema and scaling.Профиль безопасности
Poison by ingestion, intravenous, and intraperitoneal routes. Moderately toxic by subcutaneous route. An experimental teratogen. Human systemic effects by skin contact: ear tinnitus. Mutation data reported. A skin and severe eye irritant. Experimental reproductive effects. Incompatible with iron salts, spirit nitrous ether, lead acetate, iodine. Used in the manufacture of aspirin. When heated to decomposition it emits acrid smoke and irritating fumes.Возможный контакт
Used as a topical keratolytic agent; in manufacture of aspirin, salicylates, resins, as a dyestuff intermediate; prevulcanization inhibitor; analytical reagent; fungicide, antiseptic, and food preservative.Методы очистки
It has been purified by steam distillation, by recrystallisation from H2O (solubility is 0.22% at room temperature and 6.7% at 100o), absolute MeOH, or cyclohexane and by sublimation in a vacuum at 76o. The acid chloride (needles) has m 19-19.5o, b 92o/15mm, the amide has m 133o (yellow needles from H2O), the O-acetyl derivative has m 135o (rapid heating and the liquid resolidifies at 118o), and the O-benzoyl derivative has m 132o (aqueous EtOH). [IR: Hales et al. J Chem Soc 3145 1954, Bergmann et al. J Chem Soc 2351 1950]. [Beilstein 10 IV 125.]Несовместимости
iron salts; lead acetate; iodine. Forms an explosive mixture in air.Салициловая кислота запасные части и сырье
сырьё
1of2
запасной предмет
- 4-ОКТИЛФЕНИЛОВЫЙ ЭФИР салициловой кислоты
- Натрия салицилат
- 5-аминосалициловая кислота
- Этил салицилат
- C.I. Acid Black 194
- Direct Dark Brown NM
- 4-(2-(5-ХОЛРО-2-МЕТОКСИБЕНЗАМИДО)ЭТИЛ)БЕНЗОЛСУЛЬФОНИЛХЛОРИД
- Олсалазин
- Isocarbophos
- 3,5-Diiodosalicylic кислота
- Изофенфос-METHYL
- Кислотно-красный 215
- 5-ХЛОР-2-МЕТОКСИБЕНЗОЙЛХЛОРИД
- Кислота фиолетовая 68
- КИСЛОТА ацетилсалициловая
1of8
Салициловая кислота поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | ||
| +8613812269233 | China | 322 | 58 | ||
| +8617653113209 | China | 3049 | 58 | ||
| +862156762820 +86-13564624040 |
China | 7707 | 58 | ||
| +undefined-27-86652399 +undefined13627115097 |
China | 942 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-15271838296; +8615271838296 |
China | 1512 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| 0592-5800732; +8613806035118 |
China | 988 | 58 | ||
| 18758118018 | China | 255 | 58 |
Салициловая кислота Обзор)
1of4