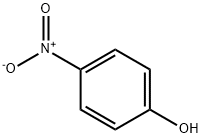
4-нитрофенол
- английское имя4-Nitrophenol
- CAS №100-02-7
- CBNumberCB7852550
- ФормулаC6H5NO3
- мольный вес139.11
- EINECS202-811-7
- номер MDLMFCD00007331
- файл Mol100-02-7.mol
| Температура плавления | 112 °C |
| Температура кипения | 279 °C(lit.) |
| Плотность накопления | 550kg/m3 |
| плотность | 1,27 g/cm3 |
| давление пара | 0.6 mm Hg ( 120 °C) |
| показатель преломления | 1.5723 (estimate) |
| Fp | 169 °C |
| температура хранения | 2-8°C |
| растворимость | ethanol: soluble95%, clear, dark yellow (100 mg/mL) |
| форма | Crystalline Powder, Crystals and/or Chunks |
| пка | 7.15(at 25℃) |
| цвет | Yellow to brown |
| Удельный вес | 1.479 |
| Запах | Odorless |
| Водородный показатель | 5.3(colorless)-7.6(Yellow) |
| РН | 4.4 (5g/l, H2O, 24℃)(anhydrous substance) |
| Скорость испарения | <1 |
| Растворимость в воде | 1.6 g/100 mL (25 ºC) |
| Чувствительный | Light Sensitive |
| λмакс | 320nm, 405nm |
| Мерк | 14,6620 |
| БРН | 1281877 |
| констант закона Генри | 1.63 x 10-7 at 5 °C (average derived from six field experiments, Lüttke and Levsen, 1997) |
| Стабильность | Stable. Incompatible with strong oxidizing agents, strong bases, organics, combustible material, reducing agents. Combustible. |
| Основное приложение | Display device, solar cells, nanoparticles, electrolytic capacitors, lithographic printing plate, leak detection method, falsification-proof security paper, correction fluid, detergent, fertilizer, identifying fresh and stale rice, diapers, detecting lactic acid bacteria, drug delivery, evaluating dental caries activity |
| Справочник по базе данных CAS | 100-02-7(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 4 |
| FDA UNII | Y92ZL45L4R |
| Справочник по химии NIST | Phenol, 4-nitro-(100-02-7) |
| Система регистрации веществ EPA | p-Nitrophenol (100-02-7) |
| Пестициды Закон о свободе информации (FOIA) | Paranitrophenol |
| UNSPSC Code | 41116107 |
| NACRES | NA.28 |
| Коды опасности | Xn,T,F | |||||||||
| Заявления о рисках | 20/21/22-33-39/23/24/25-23/24/25-11 | |||||||||
| Заявления о безопасности | 28-28A-45-36/37-16-7 | |||||||||
| РИДАДР | UN 1663 6.1/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | SM2275000 | |||||||||
| F | 8 | |||||||||
| Температура самовоспламенения | 541 °F | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29089000 | |||||||||
| Банк данных об опасных веществах | 100-02-7(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in mice, rats: 467, 616 mg/kg, K.C. Back et al., Reclassification of Materials Listed as Transportation Health Hazards (TSA-20-72-3; PB214-270, 1972) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
H302+H312+H332:Вредно при проглатывании, при попадании на кожу или при вдыхании.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
P314:В случае плохого самочувствия обратиться к врачу.
4-нитрофенол химические свойства, назначение, производство
Описание
4-Nitrophenol (also called p-nitrophenol or 4-hydroxynitrobenzene) is a phenolic compound that has a nitro group at the opposite position of hydroxyl group on the benzene ring. 4-Nitrophenol shows two polymorphs in the crystalline state. The alpha form is colorless pillars, unstable at room temperature, and stable toward sunlight. The beta form is yellow pillars, stable at room temperature, and gradually turns red upon irradiation of sunlight. Usually 4-nitrophenol exists as a mixture of these two forms. Generally, 4-nitrophenol is used in manufacturing of drugs (e.g., acetaminophen), fungicides, methyl and ethyl parathion insecticides, and dyes, and to darken leather.Химические свойства
Yellow to tan crystals or powder (mixed α- and β-forms), The (metastable) α-form crystallizes from toluene above 63°C, and the yellow, prismatic β-form crystallizes from toluene below 63°C. 4-Nitrophenol is not steam volatile and is much more soluble in water (30 % at 100°C) than the ortho isomer.Использование
4-Nitrophenol is used in dyestuff and pesticide synthesis, as a fungicide, bactericide, and wood preservative, as a chemical indicator, and as a substrate for experiments on cytochrome P450 2E1.4-Nitrophenol is used as a precursor to prepare phenetidine, acetophenetidne and pH indicator. Its carboxylate ester derivatives are involved as an active component for the construction of amide moieties in peptide synthesis. It is used as an intermediate in the synthesis of paracetamol and N-acetyl-p-aminophenol dyestuffs.
прикладной
4-Nitrophenol (4-NP) is used to manufacture pharmaceuticals, fungicides, insecticides, and dyes and to darken leather. Indicator in 0.1% alcohol solution. pH: 5.6 colorless, 7.6 yellow. It can be used to prepare 4-aminophenol (4-AP), a key intermediate for the manufacture of analgesic and antipyretic drugs.Подготовка
4-Nitrophenol was synthesized from p-nitrochlorobenzene by hydrolysis and acidification. Add 2320-2370L of sodium hydroxide solution with a concentration of 137-140g/L to the hydrolysis pot, and then add 600kg of molten p-nitrochlorobenzene. Heat to 152℃, pressure in the pot is 0.4MPa, then stop heating, the hydrolysis reaction exotherm makes the temperature and pressure rise naturally to 165℃, about 0.6MPa. keep 3h and take sample to check the end point of the reaction, after the reaction is finished, the hydrolysate is cooled to 120℃. Add 600L water and 50L concentrated sulfuric acid to the crystallization pot, press into the above hydrolysis and cool to about 50℃, add concentrated sulfuric acid to make the Congo red test paper purple, continue to cool to 30℃, filter, centrifuge to shake off the water, get more than 90% of 4-nitrophenol about 500kg, 92% yield.Определение
ChEBI: 4-nitrophenol is a member of the class of 4-nitrophenols that is phenol in which the hydrogen that is para to the hydroxy group has been replaced by a nitro group. It has a role as a human xenobiotic metabolite and a mouse metabolite. It is a conjugate acid of a 4-nitrophenolate.Общее описание
4-nitrophenol appears as a white to light yellow crystalline solid. Contact may severely irritate skin and eyes. Poisonous by ingestion and moderately toxic by skin contact.Реакции воздуха и воды
Soluble in hot water and more dense than water.Профиль реактивности
4-Nitrophenol is a slightly yellow, crystalline material, moderately toxic. Mixtures with diethyl phosphite may explode when heated. Decomposes exothermally, emits toxic fumes of oxides of nitrogen [Lewis, 3rd ed., 1993, p. 941]. Decomposes violently at 279°C and will burn even in absence of air [USCG, 1999]. Solid mixtures of the nitrophenol and potassium hydroxide (1:1.5 mol) readily deflagrate [Bretherick, 5th Ed., 1995].Опасность
Toxic by ingestion.Угроза здоровью
Acute inhalation or ingestion of 4-nitrophenol in humans causes headaches, drowsiness, nausea, and cyanosis. Contact with the eyes causes irritation.A study examining the acute effects of 4-nitrophenol from inhalation exposure in rats reported an increase in methemoglobin and corneal opacity.
Tests involving acute exposure of rats and mice have shown 4-nitrophenol to have high toxicity from oral and dermal exposure.
Профиль безопасности
4-Nitrophenol is used to manufacture drugs, fungicides, insecticides, and dyes and to darken leather. Acute (short-term) inhalation or ingestion of 4-nitrophenol in humans causes headaches, drowsiness, nausea, and cyanosis (blue color in lips, ears, and fingernails). Contact with eyes causes irritation in humans. No information is available on the chronic (long-term) effects of 4-nitrophenol in humans or animals from inhalation or oral exposure. No information is available on the reproductive, developmental, or carcinogenic effects of 4-nitrophenol in humans. EPA has not classified 4-nitrophenol for potential carcinogenicity.Синтез
4-Nitrophenol is synthesized by Nitration of para-Toluenesulfonic ester of Phenol, followed by hydrolysis is obtained a fairly pure material.Метаболический путь
4-[U-14C]Nitrophenol is conjugated as its b-glucoside (ca 22% of applied 14C) and gentiobioside, glc- b(126)-glc-b-4-nitrophenol (ca 64%), while about 7% of the parent remains unchanged in cell suspension cultures of Datura stramonium (L.). Gal-b-4-nitrophenol is found to be a minor metabolite.Методы очистки
Crystallise 4-nitrophenol from water (which may be acidified, e.g. with N H2SO4 or 0.5N HCl), EtOH, aqueous MeOH, CHCl3, *benzene or pet ether, then dry it in vacuo over P2O5 at 25o. It can be sublimed at 60o/10-4mm. The 4-nitrobenzoate had m 159o (from EtOH). [Beilstein 6 IV 1279.]4-нитрофенол запасные части и сырье
сырьё
1of2
запасной предмет
- 2,6-Dichloroindophenol sodium salt
- 2,6-дибром-4-нитрофенол
- 4-аминофенил эфир
- Бис (4-нитрофенил) карбона
- PARAOXON
- 2-Нитрофенол
- 4-NITROPHENETOLE
- 4-нитрофенил фосфат, динатриевая соль, гексагидрат
- 4-нитрофенил бета-D-галактопиранозид
- Альфа-бром-4-нитро-o-крезол
- 4-аминофенол
- 4-нитрофенил-альфа-D-глюкопиранозид
- 4-бензилоксианилин
- 2-Хлор-4-нитрофенол
- 4-Нитрофенил-^ AD-галактопиранозид
1of5
4-нитрофенол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-021-021-50872116 +8613122107989 |
China | 10035 | 58 | |
| +8618950047208 | China | 43416 | 58 | |
| +undefined+86-18049800532 | China | 2764 | 58 | |
| 025-66023220 0086-15250997978 |
China | 7892 | 55 | |
| 027-83850116 18164090116 |
China | 4264 | 58 | |
| 028-64441822 18581895003 |
China | 3337 | 58 | |
| 17717235263 | China | 17730 | 58 | |
| 027-02783261126 18971318572 |
China | 4992 | 58 | |
| 18913906212 | China | 7160 | 58 | |
| 15805316389 | China | 432 | 58 |