Фталевого ангидрида
- английское имяPhthalic anhydride
- CAS №85-44-9
- CBNumberCB7414905
- ФормулаC8H4O3
- мольный вес148.12
- EINECS201-607-5
- номер MDLMFCD00005918
- файл Mol85-44-9.mol
| Температура плавления | 131-134 °C(lit.) |
| Температура кипения | 284 °C(lit.) |
| плотность | 1,53 g/cm3 |
| плотность пара | 5.1 (vs air) |
| давление пара | <0.01 mm Hg ( 20 °C) |
| показатель преломления | 1.4500 (estimate) |
| Fp | 152 °C |
| температура хранения | Store below +30°C. |
| растворимость | 6g/l (slow decomposition) |
| форма | Flaky Crystals |
| пка | 2.97[at 20 ℃] |
| цвет | White |
| Запах | Characteristic choking odor |
| Водородный показатель | 2 at 6 g/l at 20 °C |
| РН | 2 (6g/l, H2O, 20℃) |
| Пределы взрываемости | 1.7-10.5%(V) |
| Растворимость в воде | 6 g/L (20 ºC) |
| Чувствительный | Moisture Sensitive |
| Мерк | 14,7372 |
| БРН | 118515 |
| констант закона Генри | 6.29 at 20 °C (approximate - calculated from water solubility and vapor pressure) |
| Пределы воздействия | NIOSH REL: TWA 6 mg/m3 (1 ppm), IDLH 60 mg/m3; OSHA PEL: TWA 12 mg/m3 (2 ppm); ACGIH TLV: TWA 1 ppm (adopted). |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents, strong bases, moisture, strong acids. Dust may form an explosive mixture with air. |
| LogP | 2.07 at 20℃ |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | PHTHALIC ANHYDRIDE |
| FDA 21 CFR | 175.105; 177.1460; 177.2600 |
| Справочник по базе данных CAS | 85-44-9(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 3-5 |
| FDA UNII | UVL263I5BJ |
| Справочник по химии NIST | Phthalic anhydride(85-44-9) |
| Система регистрации веществ EPA | Phthalic anhydride (85-44-9) |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 22-37/38-41-42/43 | |||||||||
| Заявления о безопасности | 23-24/25-26-37/39-46-22 | |||||||||
| РИДАДР | 2214 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 6 mg/m3 (1 ppm) | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | TI3150000 | |||||||||
| F | 10-21 | |||||||||
| Температура самовоспламенения | 580 °C | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29173500 | |||||||||
| Банк данных об опасных веществах | 85-44-9(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 1530 mg/kg LD50 dermal Rabbit > 3160 mg/kg | |||||||||
| ИДЛА | 60 mg/m3 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
H318:При попадании в глаза вызывает необратимые последствия.
H317:При контакте с кожей может вызывать аллергическую реакцию.
H334:При вдыхании может вызывать аллергическую реакцию (астму или затрудненное дыхание).
-
оператор предупредительных мер
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312+P330:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии. Прополоскать рот.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338+P310:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз. Немедленно обратиться за медицинской помощью.
Фталевого ангидрида химические свойства, назначение, производство
Описание
Phthalic anhydride is the organic compound with the formula C6H4(CO)2O. It is the anhydride of phthalic acid. This colourless solid is an important industrial chemical, especially for the large-scale production of plasticizers for plastics.

Phthalic anhydride is an important chemical intermediate in the plastics industry from which are derived numerous phthalate esters that function as plasticizers in synthetic resins. Phthalic anhydride itself is used as a monomer for synthetic resins such as glyptal, the alkyd resins, and the polyester resins.
Phthalic anhydride is also used as a precursor of anthraquinone, phthalein, rhodamine, phthalocyanine, fluorescein, and xanthene dyes.
Phthalic anhydride is used in the synthesis of primary amines, the agricultural fungicide phaltan, and thalidomide. Other reactions with phthalic anhydride yield phenolphthalein, benzoic acid, phthalylsulfathiazole (an intestinal antimicrobial agent), and orthophthalic acid.
Химические свойства
Phthalic Anhydride is moderately flammable, white solid (flake) or a clear, colorless, mobile liquid (molten) Characteristic, acrid, choking odor. It is very slightly soluble in H2O, soluble in alcohol, and slightly soluble in ether.Физические свойства
Colorless to pale cream crystals with a characteristic, choking odor. Moisture sensitive. Odor threshold concentration is 53 ppb (quoted, Amoore and Hautala, 1983).Определение
ChEBI: Phthalic anhydride is the cyclic dicarboxylic anhydride that is the anhydride of phthalic acid. It has a role as an allergen. It is a cyclic dicarboxylic anhydride and a member of 2-benzofurans.Подготовка
The most important modifying component used in the manufacture of linear unsaturated polyesters is phthalic anhydride. The anhydride is generally obtained by the oxidation of o-xylene: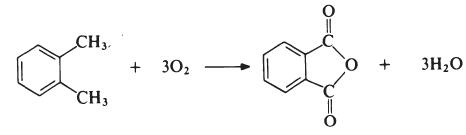
The reaction is carried out in the vapour phase by passing a mixture of o-xylene and air over a catalyst such as vanadium pentoxide supported on silica and promoted with titanium dioxide at about 400??C. The exit gases are cooled and the phthalic anhydride is collected and purified by distillation under reduced pressure.
Общее описание
A colorless to white lustrous solid in the form of needles with a mild distinctive odor. Moderately toxic by inhalation or ingestion and a skin irritant. Melting point 64°F Flash point 305°F. Forms a corrosive solution when mixed with water. Used in the manufacture of materials such as artificial resins.Реакции воздуха и воды
Reacts, usually slowly with water to form phthalic acid and heat [Merck 11th ed. 1989]. The phthalic acid is somewhat soluble in water.Профиль реактивности
Phthalic anhydride reacts exothermically with water. The reactions are sometimes slow, but can become violent when local heating accelerates their rate. Acids accelerate the reaction with water. Incompatible with acids, strong oxidizing agents, alcohols, amines, and bases. Undergoes exothmeric nitration with fuming nitric acid-sulfuric acid and may give mixtures of the potentially explosive phthaloyl nitrates or nitrites or their nitro derivatives [Chem. & Ind. 20:790. 1972]. Phthalic anhydride reacts violently with CuO at elevated temperatures [Park, Chang-Man, Richard J. Sheehan. hthalic Acids and Other Benzenepolycarboxylic Acids Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. 2005]. Mixtures of Phthalic anhydride and anhydrous CO2 explode violently if heated [eaflet No. 5, Inst. of Chem., London, 1940].Угроза здоровью
Solid irritates skin and eyes, causing coughing and sneezing. Liquid causes severe thermal burns.Пожароопасность
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.Фармацевтические приложения
Phthalic anhydride reacted with cellulose acetate forms cellulose acetate phthalate (CAP), a common enteric coating excipient that has also been shown to have antiviral activity. Phthalic anhydride is a degradation product of CAP.Контактные аллергены
Phthalic anhydride is used in the manufacture of unsaturated polyesters and as a curing agent for epoxy resins. When used as a pigment, it can be responsible for sensitization in ceramic workers. Phthalic anhydride per se is not responsible for the sensitization to the resin used in nail varnishes phthalic anhydride/trimellitic anhydride/ glycols copolymer.Профиль безопасности
Poison by ingestion. Experimental teratogenic effects. A corrosive eye, skin , and mucous membrane irritant. A common air contaminant. Combustible when exposed to heat or flame; can react with oxidzing materials. Moderate explosion hazard in the form of dust when exposed to flame. The production of ths material has caused many industrial explosions. Mixtures with copper oxide or sodium nitrite explode when heated. Violent reaction with nitric acid + sulfuric acid above 80℃. To fight fire, use CO2, dry chemical. Used in plasticizers, polyester resins, and alkyd resins, dyes, and drugs. See also ANHYDRIDES.Возможный контакт
Phthalic anhydride is used in plasticizers; in the manufacture of phthaleins; benzoic acid; alkyd and polyester resins; synthetic indigo; and phthalic acid;which is used as a plasticizer for vinyl resins. To a lesser extent, it is used in the production of alizarin, dye, anthranilic acid; anthraquinone, diethyl phthalate; dimethyl phthalate; erythrosine, isophthalic acid; methylaniline, phenolphthalein, phthalamide, sulfathalidine, and terephthalic acid. It has also found uses as a pesticide intermediate.Перевозки
UN2214 Phthalic anhydride with>.05 % maleic anhydride, Hazard class: 8; Labels: 8-Corrosive material.Методы очистки
Distil the anhydride under reduced pressure. Purify it from the acid by extracting with hot CHCl3, filtering and evaporating. The residue is crystallised from CHCl3, CCl4 or *benzene, or sublimed. Fractionally crystallise it from its melt. Dry it under vacuum at 100o. [Saltiel J Am Chem Soc 108 2674 1986, Beilstein 17/11 V 253.]Несовместимости
Dust forms an explosive mixture with air. Phthalic anhydride reacts exothermically with water. The reactions are sometimes slow, but can become violent when local heating accelerates their rate. Acids accelerate the reaction with water. Incompatible with acids, strong oxidizing agents, alcohols, amines, and bases. Converted to phthalic acid in hot water. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. caustics, ammonia, amines, water. Reacts violently with copper oxide or sodium nitrite 1 heat.Утилизация отходов
Use a licensed professional waste disposal service to dispose of this material. Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.Фталевого ангидрида запасные части и сырье
сырьё
1of3
запасной предмет
- Диаллилфталатная
- Potassium hydrogen phthalate
- 1,2,5-оксадиaзол-3-карбоновая кислота
- N-(Гидроксиметил)фталимид
- Н-ФТАЛОИЛ-DL-ГЛУТАМИНОВЫЙ АНГИДРИД 98
- Родамин B основн.
- Alkyd resin insulating paint
- 2',7'-dibromo-3',6'-dihydroxyspiro[isobenzofuran-1(3H),9'-[9H]xanthene]-3-one
- ДИ-ИЗО-ДЕЦИЛФТАЛАТ
- 2-БЕНЗОИЛБЕНЗОИЛХЛОРИД
- N-PHENYLPHTHALIMIDE
- 2-Bibenzylcarboxylic acid
- 1-ХЛОРО-4-МЕТОКСИПТАЛАЗИН
- 2-Chloroanthraquinone
- 5-(2-Carboxybenzoyl)-2-chlorobenzenesulfonyl chloride
- DIPHENYL PHTHALATE
- Дипентил фталат
- Amino resin varnish
- 2,3,4,5-Тетрафторбензойная кислота
- Динонил фтала
1of8
Фталевого ангидрида поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8617320528784 | China | 41 | 58 | ||
| +86-13131129325 | China | 5867 | 58 | ||
| +8615530197691 | China | 105 | 58 | ||
| +86-021-62885108 +8613917661608 |
China | 2068 | 57 | ||
| 571-85586718 +8613336195806 |
China | 29798 | 60 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21667 | 55 | ||
| +undefined-21-51877795 | China | 32836 | 60 | ||
| +86-0551-65418679 +8618949832763 |
China | 2989 | 55 | ||
| +86-13734021967 +8613734021967 |
China | 1011 | 58 | ||
| +86-0371-86658258 +8613203830695 |
China | 29889 | 58 |