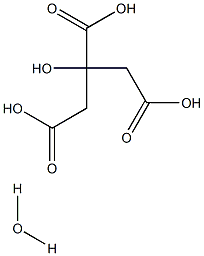
Лимонная кислота моногидрат
- английское имяCitric acid monohydrate
- CAS №5949-29-1
- CBNumberCB3235573
- ФормулаC6H10O8
- мольный вес210.1388
- EINECS200-662-2
- номер MDLMFCD00008765
- файл Mol5949-29-1.mol
| Температура плавления | -94 °C(lit.) |
| Температура кипения | 56 °C760 mm Hg(lit.) |
| Плотность накопления | 800-1000kg/m3 |
| плотность | 0.791 g/mL at 25 °C(lit.) |
| плотность пара | 2 (vs air) |
| давление пара | 184 mm Hg ( 20 °C) |
| показатель преломления | n |
| Fp | 1 °F |
| температура хранения | no restrictions. |
| растворимость | Citric Acid Monohydrate is very soluble in water, freely soluble in ethanol and sparingly soluble in ether. |
| форма | Solid |
| пка | 3.138, 4.76, 6.401 |
| Удельный вес | 0.810 (20/4℃) |
| цвет | White |
| РН | 1.85 (50g/l, H2O, 25℃) |
| Запах | Typical, practically odorless |
| Растворимость в воде | 1630 g/L (20 oC) ;H2O: soluble 54% (w/w) at 10°C (Citric acid in water) |
| Чувствительный | Hygroscopic |
| Мерк | 14,2326 |
| БРН | 4018641 |
| Стабильность | Stable. Incompatible with oxidizing agents, bases, reducing agents, nitrates. |
| ИнЧИКей | YASYEJJMZJALEJ-UHFFFAOYSA-N |
| Справочник по базе данных CAS | 5949-29-1(CAS DataBase Reference) |
| FDA UNII | 2968PHW8QP |
| Справочник по химии NIST | Citric acid monohydrate(5949-29-1) |
| Система регистрации веществ EPA | 1,2,3-Propanetricarboxylic acid, 2-hydroxy-, hydrate (1:1) (5949-29-1) |
| UNSPSC Code | 41116107 |
| NACRES | NA.25 |
| Коды опасности | F,Xi | |||||||||
| Заявления о рисках | 11-36-66-67-41-36/37/38-37/38 | |||||||||
| Заявления о безопасности | 9-16-26-37/39-36/37/39-36 | |||||||||
| РИДАДР | UN 1090 3/PG 2 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | AL3150000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29181400 | |||||||||
| Токсичность | LD50 orally in Rabbit: 3000 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P264:После работы тщательно вымыть кожу.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Лимонная кислота моногидрат химические свойства, назначение, производство
Химические свойства
Citric acid monohydrate occurs as colorless or translucent crystals or as a white crystalline, efflorescent powder. It is odorless and has a strong acidic taste. The crystal structure is orthorhombic. monohydrate crystals lose water of crystallization in dry air or when heated to about 40 to 50℃. Citric acid monohydrate softens at 75℃ and melts at approximately 100℃. It serves as a natural preservative and is commonly utilized to contribute an acidic or sour taste to foods and soft drinks.
Citric acid monohydrate acts as a preservative and antioxidant. It is also used as an acidulant, flavoring agent and antistaling agent in fruit drinks, candy, cookies, biscuits, canned fruits, jams, and jellies. It differs from other forms of citric acid by having a moisture percentage ranging from 7.5-9.0.
Использование
Citric Acid Monohydrate is a tricarboxylic acid found in citrus fruits. Citric acid is used as an excipient in pharmaceutical preparations due to its antioxidant properties. It maintains the stability of active ingredients and is used as a preservative. It is also used as an acidulant to control pH and acts as an anticoagulant by chelating calcium in the blood. Additionally, Citric Acid Monohydrate is used as an acidulating, food additive, and synergist in antioxidant mixtures.прикладной
Citric acid monohydrate has multiple applications. It is used for preparing citrate buffer in platelets for intravital microscopy and as a buffer component for unmasking antigens and epitopes. Additionally, it functions as a pH-control agent in food, beverage, and pharmaceutical industries. In animals, it enhances the efficiency of nutritional calcium utilization.Recent uses of citric acid monohydrate include:
As a carbon source in carbon nanodot synthesis
To prepare a pH-6.0 citric acid monohydrate solution/buffer for tissue sample preparation
In citric acid solubilization technique to treat various samples.
Подготовка
Currently, the industrial production process for citric acid monohydrate crystals involves several steps. First, the citric acid fermentation liquid is separated from the solid-liquid to obtain a citric acid clear liquid. Then, the clear liquid undergoes purification via the calcium salt method (hydrogen calcium method), acidolysis, and decolorization. This purified solution is then heated, concentrated, and crystallized to produce anhydrous citric acid. The mother liquor remaining from this process is then filtered by plate and frame and added to a crystallization cylinder to reach a supersaturated state through cooling and precipitating the crystal. Once the temperature reaches around 13 ℃, the mixture undergoes centrifugal separation, and the resulting wet crystal is then dried in a dryer. The separated mother liquor, after being filtered, decolorized, and concentrated, is added back to the crystallization cylinder to repeat the above steps a total of four to five times. Finally, the mother liquor is returned to the extraction workshop for re-purification via chromatographic separation.Общее описание
Citric acid monohydrate is an organic acid. Its molar enthalpy of solution in water has been reported to be ΔsolHm (298.15K, m = 0.0203molkg-1) = (29061 ± 123)Jmol-1Безопасность
Citric acid monohydrate is non-toxic and has a low reactivity.May cause gastrointestinal irritation with nausea, vomiting and diarrhea. Excessive intake of citric acid may cause erosion of the teeth. Inhalation: Causes respiratory tract irritation. Chronic: Repeated exposure may cause erosion of teeth.
хранилище
Citric acid monohydrate loses water of crystallization in dry air or when heated to about 408℃. It is slightly deliquescent in moist air. Dilute aqueous solutions of citric acid may ferment on standing.Методы очистки
Crystallise it from hot H2O solution (w/w solubility is 54% at 10o, 71% at 50o and 84% at 100o. The monohydrate (softens at ~75o and melts at ~100o) dehydrates in air or when heated gently above 40o . The triethylester ( M 276.3, b 127o/1mm, 294o/atm, d 4 1.137, n D 1.4420.) is a bitter tasting oil. [Beilstein 3 H 556 and 568, 3 IV 1272.]Несовместимости
Citric acid is incompatible with potassium tartrate, alkali and alkaline earth carbonates and bicarbonates, acetates, and sulfides. Incompatibilities also include oxidizing agents, bases, reducing agents, and nitrates. It is potentially explosive in combination with metal nitrates. On storage, sucrose may crystallize from syrups in the presence of citric acid.Регуляторный статус
GRAS listed. The anhydrous form is accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (inhalations; IM, IV, and other injections; ophthalmic preparations; oral capsules, solutions, suspensions and tablets; topical and vaginal preparations). Included in nonparenteral and parenteral medicines licensed in Japan and the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.использованная литература
A comparison of properties between the citric acid monohydrate and deep eutectic solvent extracted Averrhoa bilimbi pectins DOI:10.1007/s11694-020-00533-xMethods of multilocus enzyme electrophoresis for bacterial population genetics and systematics DOI:10.1128/aem.51.5.873-884.1986
Increased Adhesion and Aggregation of Platelets Lacking Cyclic Guanosine 3′,5′-Monophosphate Kinase I DOI:10.1084/JEM.189.8.1255
Citric Acid, Anhydrous, and Citric Acid, Monohydrate DOI: 10.1021/acsreagents.4096.20211101
Production method for citric acid monohydrate crystal
https://pubchem.ncbi.nlm.nih.gov/compound/22230
Лимонная кислота моногидрат запасные части и сырье
Лимонная кислота моногидрат поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8615350851019 | China | 1001 | 58 | ||
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | ||
| +8613031735486 | China | 105 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-027-59207850 | China | 5972 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +8613043004613 | China | 283 | 58 |