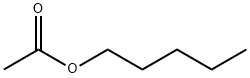
Амилацетат
- английское имяAmyl acetate
- CAS №628-63-7
- CBNumberCB7166381
- ФормулаC7H14O2
- мольный вес130.18
- EINECS211-047-3
- номер MDLMFCD00009500
- файл Mol628-63-7.mol
| Температура плавления | −100 °C(lit.) |
| Температура кипения | 142-149 °C(lit.) |
| плотность | 0.876 g/mL at 25 °C(lit.) |
| плотность пара | 4.5 (vs air) |
| давление пара | 4 mm Hg ( 20 °C) |
| показатель преломления | n |
| Fp | 75 °F |
| температура хранения | Store below +30°C. |
| растворимость | 10g/l |
| форма | Powder |
| цвет | White |
| Запах | Pleasant banana-like; mild; characteristic banana- or pear-like odor. |
| Пределы взрываемости | 1.1-7.5%(V) |
| Биологические источники | synthetic |
| Odor Type | fruity |
| Растворимость в воде | 10 g/L (20 ºC) |
| Точка замерзания | -70.8℃ |
| БРН | 1744753 |
| констант закона Генри | 10.73 at 37 °C (static headspace-GC, Bylaite et al., 2004) |
| Пределы воздействия | TLV-TWA 100 ppm (~525 mg/m3) (ACGIH, MSHA, and OSHA); IDLH 4000 ppm. |
| Диэлектрическая постоянная | 5.0(20℃) |
| LogP | 2.300 |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | AMYL ACETATE |
| FDA 21 CFR | 175.105 |
| Справочник по базе данных CAS | 628-63-7(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 92Q24NH7AS |
| Справочник по химии NIST | Acetic acid, pentyl ester(628-63-7) |
| Система регистрации веществ EPA | n-Amyl acetate (628-63-7) |
| UNSPSC Code | 85151701 |
| NACRES | NB.21 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 10-66 | |||||||||
| Заявления о безопасности | 23-25-24/25 | |||||||||
| РИДАДР | UN 1104 3/PG 3 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 100 ppm (525 mg/m3) | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | AJ1925000 | |||||||||
| F | 21 | |||||||||
| Температура самовоспламенения | 680 °F &_& 714 °F | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29153930 | |||||||||
| Банк данных об опасных веществах | 628-63-7(Hazardous Substances Data) | |||||||||
| Токсичность | Acute oral LD50 for rats 6,500 mg/kg (quoted, RTECS, 1985). | |||||||||
| ИДЛА | 1,000 ppm | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H226:Воспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P233:Держать в плотно закрытой/герметичной таре.
P240:Заземлить и электрически соединить контейнер и приемное оборудование.
P241:Использовать взрывобезопасное оборудование и освещение.
P242:Использовать неискрящие приборы.
P243:Принимать меры предосторожности против статических разрядов.
Амилацетат химические свойства, назначение, производство
Химические свойства
All isomers of amyl acetate are highly flammable, colorless to yellow, watery liquids.Физические свойства
Colorless liquid with a sweet, banana-like odor. A detection odor threshold concentration of 275 μg/m3 (52 ppbv) was reported by Punter (1983). Cometto-Mu?iz and Cain (1991) reported an average nasal pungency threshold concentration of 1,650 ppmv.Использование
A colorless liquid made by adding sulfuric acid to a mixture of amyl alcohol and acetic acid with subsequent recovery by distillation. It is slightly soluble in water but insoluble in alcohol. Amyl acetate was used as one of the solvents in making celluloid film and as fuel for the Alteneck lamp, adopted as the standard light in sensitometry by the International Congress of Photography in 1889.Методы производства
n-Amyl acetate is the produced by the esterification of N-amyl alcohol with acetic acid.Определение
ChEBI: An acetate ester of pentanol.Общее описание
A mixture of isomers. A clear colorless liquid with a banana-like odor. Flash point varies from 65°F. to 95°F. Less dense (at 7.2 lb / gal) than water and slightly soluble in water. Hence floats on water. Vapors heavier than air.Реакции воздуха и воды
Highly flammable. Slightly soluble in water.Профиль реактивности
AMYL ACETATE is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Amyl acetate is incompatible with the following: Nitrates; strong oxidizers, alkalis & acids .Опасность
Flammable, high fire risk. Explosive limits in air 1.1–7.5%.Угроза здоровью
n-Amyl acetate is a narcotic, an irritant tothe eyes and respiratory passage, and at highconcentrations, an anesthesia. Exposure toabout 300 ppm in air for 30 minutes mayproduce eye irritation in humans. Higherconcentrations (>1000 ppm) may produceheadache, somnolence, and narcotic effects.Exposure to 5200 ppm for 8 hours was lethalto rats. It is more toxic than the loweraliphatic esters. An LD50 value in rats iswithin the range 6000 mg/kg.Пожароопасность
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.Химическая реактивность
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Профиль безопасности
Moderately toxic by intraperitoneal route. Human systemic effects by inhalation: conjunctiva irritation, headache, and somnolence. A human eye irritant. Apparently more toxic than butyl acetate. Chronic toxicity is of a low order. Dangerous fire hazard when exposed to heat or flame; can react with oxidizing materials. Moderately explosive in the form of vapor when exposed to flame. To fight fire, use alcohol foam, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS, AMYL ALCOHOL, and ACETIC ACID.Возможный контакт
(n-isomer): Primary irritant (w/o allergic reaction), (sec-isomer) Human Data. Amyl acetates are used as industrial solvents and in the manufacturing and dry-cleaning industry; making artificial fruit-flavoring agents; cements, coated papers, lacquers; in medications as an inflammatory agent; pet repellents, insecticides and miticide. Many other uses.Канцерогенность
Not listed by ACGIH, California Proposition 65, IARC, NTP, or OSHA.Экологическая судьба
Chemical/Physical. Hydrolyzes in water forming acetic acid and 1-pentanol.At an influent concentration of 985 mg/L, treatment with GAC resulted in an effluent concentration of 119 mg/L. The adsorbability of the carbon used was 175 mg/g carbon (Guisti et al., 1974).
Перевозки
UN1993 Flammable liquids, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, Technical Name Required.Методы очистки
Shake the ester with saturated NaHCO3 solution until neutral, washed it with water, dry with MgSO4 and distil it. The ester has also been purfied by repeated fractional distillation through an efficient column or spinning band column. [Timmermann & Hennant-Roland J Chim Phys 52 223 1955, Mumford & Phillips J Chem Soc 75 1950, 1H NMR: Crawford & Foster Can J Phys 34 653 1956, Beilstein 2 IV 152.]Несовместимости
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, nitrates. May soften certain plastics.Утилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.Амилацетат запасные части и сырье
Амилацетат поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +8617531153977 | China | 5855 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-0532-86867058 +86-18562606086 |
China | 478 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 |