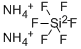
Аммоний гексафторсилика
- английское имяAmmonium hexafluorosilicate
- CAS №16919-19-0
- CBNumberCB0691538
- ФормулаF6H8N2Si
- мольный вес178.15
- EINECS240-968-3
- номер MDLMFCD00010887
- файл Mol16919-19-0.mol
| Температура плавления | °Cd ec.) |
| плотность | 2.01 g/mL at 25 °C(lit.) |
| давление пара | 9.99Pa at 20℃ |
| растворимость | water solubility 18 g/100 g water. |
| форма | Crystalline Powder and Chunks |
| Удельный вес | 2.011 |
| цвет | White |
| Растворимость в воде | Slightly soluble in water. Insoluble in alcohol and acetone. |
| Гидролитическая чувствительность | 0: forms stable aqueous solutions |
| Мерк | 14,527 |
| Пределы воздействия | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| InChI | InChI=1S/F6Si.2H3N/c1-7(2,3,4,5)6;;/h;2*1H3/q-2;;/p+2 |
| ИнЧИКей | ITHIMUMYFVCXSL-UHFFFAOYSA-P |
| SMILES | [Si-2](F)(F)(F)(F)(F)F.[NH4+].[NH4+] |
| LogP | -6.01 at 20℃ |
| Справочник по базе данных CAS | 16919-19-0(CAS DataBase Reference) |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | AMMONIUM HEXAFLUOROSILICATE |
| FDA 21 CFR | 175.105 |
| Рейтинг продуктов питания EWG | 2-3 |
| FDA UNII | 1X545W620G |
| Система регистрации веществ EPA | Ammonium silicofluoride (16919-19-0) |
| UNSPSC Code | 12352302 |
| NACRES | NA.23 |
| Коды опасности | T | |||||||||
| Заявления о рисках | 23/24/25 | |||||||||
| Заявления о безопасности | 26-45 | |||||||||
| РИДАДР | UN 2854 6.1/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | VV7800000 | |||||||||
| Примечание об опасности | Toxic | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 2826908090 | |||||||||
| Банк данных об опасных веществах | 16919-19-0(Hazardous Substances Data) | |||||||||
| Токсичность | LD orally in guinea pigs: 150 mg/kg (Simonin, Pierron) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H301+H311+H331:Токсично при проглатывании, при контакте с кожей или при вдыхании.
-
оператор предупредительных мер
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P310+P330:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
P304+P340+P311:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью.
Аммоний гексафторсилика химические свойства, назначение, производство
Химические свойства
Ammonium hexafluorosilicate is a white crystalline powder. Odorless.Физические свойства
d 2.01 g cm?3; decomposes prior to melting; odorless.Использование
Ammonium hexafluorosilicate is used as a disinfectant and analytical reagent. It finds application in etching glass, metal casting, and electroplating. It is also employed as a wood preservative agent and mothproofing agent in textiles. It plays a useful application in soldering flux. It is also used to arrest caries treatment without discoloration on demineralized primary tooth enamel.Подготовка
By reacting crude quartz with ammonium fluoride or ammonium hydrodifluoride or a mixture thereof in an aqueous medium when heated from 50 ° to 110 ° C. to form ammonium hexafluorosilicate, separating it from unreacted quartz and insoluble impurities by known methods (filtration or centrifugation) and subsequent concentration and cooling of the mother liquor for crystallization of Ammonium hexafluorosilicate.Общее описание
A white crystalline solid. Noncombustible. Corrodes aluminum. Used as a disinfectant, in etching glass, metal casting, and electroplating.Реакции воздуха и воды
Water soluble.Профиль реактивности
Salts, basic, such as Ammonium hexafluorosilicate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.Опасность
Very toxic; fatal.Угроза здоровью
Inhalation of dust can cause pulmonary irritation and can be fatal in some cases. Ingestion may be fatal. Contact with dust causes irritation of eyes and irritation or ulceration of skin.Пожароопасность
Special Hazards of Combustion Products: Toxic and irritating hydrogen fluoride, silicon tetrafluoride, and oxides of nitrogen may form in fires.Возможный контакт
This material is used as a pesticide and miticide, wood preservative, soldering flux; light metal casting; and in the etching of glass.Перевозки
UN2856 Fluorosilicates, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.Методы очистки
Crystallise the salt from water (2mL/g). After 3 recrystallisations, the Technical grade salt has Li, Na, K and Fe at 0.3, 0.2, 0.1 and 1.0 ppm respectively.Несовместимости
Aqueous solution is highly corrosive. Contact with acids reacts to form hydrogen fluoride, which is a highly corrosive and forms a toxic gas. Corrosive to aluminum. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Ammonium fluorosilicate reacts with water to form hydrofluoric acid, a source of fluoride ions. Unlike other halide ions, the fluoride ion is highly reactive, acting as a weak base and participating in some unique reactions. In particular, fluorides react strongly with compounds containing calcium, magnesium, or silicon ions, which means that solutions containing soluble fluorides are corrosive to both living tissue and glass. Hydrofluoric acid can cause severe chemical burns and is one of the few materials that can etch glass. It is also a toxic gas in its anhydrous form.Утилизация отходов
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration.Аммоний гексафторсилика запасные части и сырье
Аммоний гексафторсилика поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +1-5166625404; | United States | 20405 | 58 | ||
| +86-29-81148696 +86-17392712697 |
China | 3889 | 58 | ||
| +8613288715578 | China | 12554 | 58 | ||
| +8618531123677 | China | 5853 | 58 | ||
| +8615531151365 | China | 18126 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20202 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21622 | 55 | ||
| +undefined-21-51877795 | China | 33024 | 60 | ||
| 0086-577-64498589 | CHINA | 998 | 58 | ||
| 18871490254 | CHINA | 28172 | 58 |