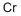
Хром
- английское имяChromium
- CAS №7440-47-3
- CBNumberCB7854190
- ФормулаCr
- мольный вес52
- EINECS231-157-5
- номер MDLMFCD00010944
- файл Mol7440-47-3.mol
| Температура плавления | 1857 °C (lit.) |
| Температура кипения | 2672 °C (lit.) |
| плотность | 7.14 g/mL at 25 °C (lit.) |
| Fp | 50 °F |
| температура хранения | no restrictions. |
| растворимость | reacts with dilute acid solutions |
| форма | powder |
| цвет | Silver-gray |
| Удельный вес | 7.2 |
| РН | <1 (H2O, 20°C) |
| Запах | Odorless |
| Flame Color | Silvery white |
| удельное сопротивление | 12.7 μΩ-cm, 20°C |
| Растворимость в воде | Insoluble in water. |
| Мерк | 13,2252 |
| Пределы воздействия | TLV-TWA: chromium metal 0.5 mg/m3 (ACGIH and MSHA), 1 mg/m3 (OSHA); Cr(II) and Cr(III) compounds 0.5 mg/m3 (ACGIH); Cr(VI) compounds, water soluble and certain water insoluble, 0.05 mg/m3 (ACGIH). |
| Стабильность | Stable. Incompatible with carbonates, strong bases, mineral acids, lithium, sulfur dioxide, strong acids. |
| Стандарт первичной питьевой воды EPA | MCL:0.1,MCLG:0.1 |
| FDA 21 CFR | 101.9; 165.110 |
| Справочник по базе данных CAS | 7440-47-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1-6 |
| FDA UNII | 0R0008Q3JB |
| Предложение 65 Список | Chromium (hexavalent compounds) |
| МАИР | 3 (Vol. Sup 7, 49) 1990 |
| Справочник по химии NIST | Chromium(7440-47-3) |
| Система регистрации веществ EPA | Chromium (7440-47-3) |
| UNSPSC Code | 41116116 |
| NACRES | NA.24 |
| Коды опасности | F,C,Xn,Xi | |||||||||
| Заявления о рисках | 11-20/21/22-34-40-23-67-36 | |||||||||
| Заявления о безопасности | 16-26-36/37/39-45-36/37-27 | |||||||||
| РИДАДР | UN 2924 3/PG 2 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 0.5 mg/m3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | GB4200000 | |||||||||
| Температура самовоспламенения | 580°C | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 8112 21 90 | |||||||||
| Класс опасности | 4.1 | |||||||||
| Группа упаковки | III | |||||||||
| Банк данных об опасных веществах | 7440-47-3(Hazardous Substances Data) | |||||||||
| Токсичность | Elemental chromium and certain chromium compounds have been designated as carcinogens, hazardous substances, hazardous waste constituents, and priority toxic pollutants. Some of those compounds designated as hazardous are chromic acetate, chromic acid, chromic sulfate, and chromous chloride. Although chromium in the 6+ state is regarded as being the most carcinogenic, there are 6+ compounds that appear to be non-carcinogenic. In addition to their possible carcinogenicity, chromium compounds may have local allergic effects leading to dermatitis. Systemically, 6+ chromium compounds are irritants to the respiratory system and may give rise to pulmonary edema. | |||||||||
| ИДЛА | 250 mg Cr/m3 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
предупреждение
-
вредная бумага
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H228:Воспламеняющееся твердое вещество.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P240:Заземлить и электрически соединить контейнер и приемное оборудование.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P405:Хранить в недоступном для посторонних месте.
Хром химические свойства, назначение, производство
Описание
Chromium as a metallic element was first discovered over 200 years ago, in 1797. But the history of chromium really began several decades before this. In 1761, in the Beresof Mines of the Ural Mountains, Johann Gottlob Lehmann obtained samples of an orange-red mineral, which he called ‘Siberian red lead.’ He analyzed this mineral in 1766 and discovered that it contained lead “mineralized with a selenitic spar and iron particles.” The mineral he found was crocoite, a lead chromate (PbCrO4).Химические свойства
Chromium may exist in one of three valence states in compounds, , , and . The most stable oxidation state is trivalent chromium; Hexavalent chromium is a less stable state. Chromium (element) blue-white to steel-gray, lustrous, brittle, hard, odorless solid. Elemental:Физические свойства
Chromium is a silvery white/gray, hard, brittle noncorrosive metal that has chemical andphysical properties similar to the two preceding elements in period 4 (V and Ti). As one of thetransition elements, its uses its M shell rather than its outer N shell for valence electrons whencombining with other elements. Its melting point is 1,857°C, its boiling point is 2,672°C,and its density is 7.19 g/cm3.Изотопы
There are 26 isotopes of the element chromium; four are stable and foundin nature, and the rest are artificially produced with half-lives from a few microsecondsto a few days. The four stable isotopes and their percentage of contribution to thetotal amount of chromium on Earth are as follows: 50Cr = 4.345%, 52Cr = 83.789%,53Cr = 9.501%, and 54Cr = 2.365%. Cr-50 is radioactive but has such a long halflife—1.8×10+17 years—that it is considered to contribute about 4% to the total amount ofchromium found on Earth.Происхождение имени
From the Greek word chroma or chromos, meaning “color,” because of the many colors of its minerals and compounds.Вхождение
Chromium is the 21st most common element found in the Earth’s crust, and chromiumoxide (Cr2O3) is the 10th most abundant of the oxide compounds found on Earth. It is notfound in a free metallic state.The first source of chromium was found in the mineral crocoite. Today it is obtained fromthe mineral chromite (FeCr2O4), which is found in Cuba, Zimbabwe, South Africa, Turkey,Russia, and the Philippines. Chromite is an ordinary blackish substance that was ignored formany years. There are different grades and forms of chromium ores and compounds, based onthe classification of use of the element. Most oxides of chromium are found mixed with othermetals, such as iron, magnesium, or aluminum.Astronauts found that the moon’s basalt rocks contain several times more chromium thanis found in basalt rocks of Earth.Характеристики
Chromium is a hard, brittle metal that, with difficulty, can be forged, rolled, and drawn,unless it is in a very pure form, in which case the chromium is easier to work with. It is anexcellent alloying metal with iron. Its bright, silvery property makes it an appropriate metal toprovide a reflective, non-corrosive attractive finish for electroplating.Various compounds of chromium exhibit vivid colors, such as red, chrome green, andchromate yellow, all used as pigments.Использование
Chromium is used in the manufacture ofits alloys, such as chrome-steel or chromenickel-steel. It is also used for chromeplatingof other metals, for tanning leather,and in catalysts. It occurs in chromite ores(FeO·Cr2O3).Методы производства
Chromium metal is prepared by reducing the ore in a blast furnace with carbon (coke) or silicon to form an alloy of chromium and iron called ferrochrome, which is used as the starting material for the many iron-containing alloys that employ chromium. Chromium to be used in iron-free alloys is obtained by reduction or electrolysis of chromium compounds.Chromiumisdif?culttoworkinthepuremetalform; it is brittle at low temperatures, and its high melting point makes it dif?cult to cast.Определение
chromium: Symbol Cr. A hard silverytransition element; a.n. 24;r.a.m. 52.00; r.d. 7.19; m.p. 1857°C;b.p. 2672°C. The main ore ischromite (FeCr2O4). The metal has abody-centred-cubic structure. It is extractedby heating chromite withsodium chromate, from whichchromium can be obtained by electrolysis.Alternatively, chromite can be heated with carbon in an electricfurnace to give ferrochrome, whichis used in making alloy steels. Themetal is also used as a shiny decorativeelectroplated coating and in themanufacture of certain chromiumcompounds.At normal temperatures the metalis corrosion-resistant. It reacts withdilute hydrochloric and sulphuricacids to give chromium(II) salts.These readily oxidize to the more stablechromium(III) salts. Chromiumalso forms compounds with the +6oxidation state, as in chromates,which contain the CrO42- ion. The elementwas discovered in 1797 byVauquelin.
Общее описание
Very hard gray solid with a metallic luster.Реакции воздуха и воды
May be pyrophoric, as dust. Insoluble in water.Профиль реактивности
Chromium reacts violently with NH4NO3, N2O2, Li, NO, KClO3, SO2 . Metal dusts when suspended in atmospheres of carbon dioxide may ignite and explode.Опасность
Hexavalent chromium compounds are questionable carcinogens and corrosive on tissue, resulting in ulcers and dermatitis on prolonged contact.Пожароопасность
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.Возможный контакт
Chromium metal is used in stainless and other alloy steels to impart resistance to corrosion, oxidation, and for greatly increasing the durability of metals; for chrome plating of other metals.Канцерогенность
Exposure to chromium compounds over a prolonged period has been observed in manyepidemiologicalstudiestoenhancetheriskofcancerof the respiratory organs among the exposed. The relationshipbetweenemploymentinindustriesproducingchromium compounds from chromite ore and enhanced risk of lungcancer iswell established.There isagreement inseveral studies that long-term exposure to some chromium-based pigments enhance the risk of lung cancer. An association has alsobeenobservedbetweenexposuretochromicacidinhard plating and lung cancer, but that association is not strong. Somestudieshaveweaklyindicatedexcessesofcancerofthe GItract,buttheresultsareinconsistentandarenotcon?rmed inwell-designedstudies.Thereisnoindicationthatchromite ore does have an associated enhanced risk of cancer. Although it has not yet been identi?ed which chromium compound (or compounds) is (are) responsible for enhanced risk of cancer in respiratory organs, there is general agreementthatitisthechromium(6+)speciesthatareresponsible for the elevated cancer risks and that the chromium species are not.Экологическая судьба
Chromium is distributed to the air, water, and soil from natural and anthropogenic sources. The environmental fate of chromium is dependent on the oxidation state and solubility of the compound and the environmental conditions affecting reduction or oxidation, such as pH. Oxidizing conditions favor the formation of Cr(VI) compounds, particularly at higher temperatures, while reducing conditions favor the formation of Cr(III) compounds. Chemical manufacturing and natural gas, oil, and gas combustion are the primary sources of chromium in the atmosphere.Most of the chromium in air eventually ends up in water or soil. Electroplating, textile manufacturing, cooling water, and leather tanning are major sources of chromium in wastewater discharges to surface waters.Chromium(III) is the predominant oxidation state of chromium in many soils. Cr(III) binds to soil and has low mobility. A lower soil pH favors the reduction of Cr(VI) to Cr(III). Runoff from soil and industrial processes may transport chromium to surface water.Cr(VI) compounds may leach into groundwater. The pH of the soil and aquatic environment is an important factor in chromium mobility, bioavailability, and toxicity. The chromate form predominates in most natural surface waters that are basic or neutral. The hydrochromate concentration increases in more acidic conditions.
Перевозки
UN3089 Metal powders, flammable, n.o.s., Hazard Class: 4.1; Labels: 4.1-Flammable solid. UN1759 Corrosive solids, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name requiredСтруктура и конформация
Two types of chromium crystals, α and β, are obtained depending on the growth method. The β type is semi-stable. It changes to a type above 800℃. The space lattice of β-Cr belongs to the hexagonal system, and its closely-packed hexagonal lattice has lattice constants of a=0.272 nm and c=0.442 nm. The space lattice of α-Cr belongs to the cubic system, and its body-centered cubic lattice has a lattice constant of a=0.28796 nm (18℃).Несовместимости
Dust may be pyrophoric in air. Chromium metal (especially in finely divided or powder form) and insoluble salts reacts violently with strong oxidants, such as hydrogen peroxide, causing fire and explosion hazard. Reacts with diluted hydrochloric and sulfuric acids. Incompatible with alkalis and alkali carbonatesУтилизация отходов
Recovery and recycling is a viable alternative to disposal for chromium in plating wastes; tannery wastes; cooling tower blowdown water and chemical plant wastes.Хром запасные части и сырье
Хром поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +undefined17712220823 | China | 615 | 58 | |
| +86-17736087130 +86-18633844644 |
China | 994 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +8615531157085 | China | 8804 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 30233 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 |