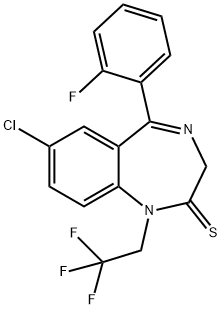
Квазепам
- английское имяQUAZEPAM
- CAS №36735-22-5
- CBNumberCB6500562
- ФормулаC17H11ClF4N2S
- мольный вес386.79
- EINECS253-179-4
- номер MDLMFCD00868025
- файл Mol36735-22-5.mol
химическое свойство
| Температура плавления | 137.5-139° |
| Температура кипения | 429.9±55.0 °C(Predicted) |
| плотность | 1.42±0.1 g/cm3(Predicted) |
| температура хранения | -20°C |
| форма | liquid |
| пка | 0.49±0.20(Predicted) |
| Справочник по базе данных CAS | 36735-22-5(CAS DataBase Reference) |
| FDA UNII | JF8V0828ZI |
| Справочник по химии NIST | Quazepam(36735-22-5) |
| Предложение 65 Список | Quazepam |
| Код УВД | N05CD10 |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| РИДАДР | 3249 |
| Класс опасности | 6.1(b) |
| Группа упаковки | III |
| кода HS | 2933996500 |
| Токсичность | LD50 in mice (mg/kg): >1370 i.v., >5000 orally (Orgini); LD50 in male, female mice, rats (mg/kg): 845, 921, 3072, 2749 i.p. (Black) |
Квазепам химические свойства, назначение, производство
Описание
Quazepam is a rapidly acting, short term hypnotic reported to exhibit a low incidence of side effects.Использование
Quazepam is a benzodiazepine derivative drug and is known to bind nonspecifically to benzodiazepine receptors, which affects muscle relaxation, anticonvulsant activty, motor coordination and memory. Quazepam is known to induce motor impairment and exerts hypnotic properties. Quazepam is often used for the treatment of insomina.Общее описание
Quazepam (Doral) and its active metabolitesreportedly are relatively selective for the benzodiazepinemodulatory site on GABAA receptors with α1 subunitand are hypnotic agents. Quazepam is metabolized by oxidation to the 2-oxo compound and then N-dealkylation.Both metabolites are active; the first reportedly is the morepotent and selective. Thereafter, 3-hydroxylation and glucuronidationoccur.