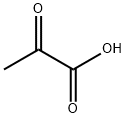
ПировиноGRадная кислота
- английское имяPyruvic acid
- CAS №127-17-3
- CBNumberCB5257557
- ФормулаC3H4O3
- мольный вес88.06
- EINECS204-824-3
- номер MDLMFCD00002585
- файл Mol127-17-3.mol
| Температура плавления | 11-12 °C (lit.) |
| Температура кипения | 165 °C (lit.) |
| плотность | 1.267 g/mL at 25 °C (lit.) |
| давление пара | 1.72hPa at 25℃ |
| FEMA | 2970 | PYRUVIC ACID |
| показатель преломления | n |
| Fp | 183 °F |
| температура хранения | 2-8°C |
| растворимость | Miscible with chloroform and methanol. |
| пка | 2.39(at 25℃) |
| форма | Liquid |
| цвет | Clear colorless to light yellow or amber |
| РН | 3.11(1 mM solution);2.38(10 mM solution);1.79(100 mM solution); |
| Запах | at 1.00 % in propylene glycol. sharp sour acetic caramellic |
| Odor Type | acidic |
| Биологические источники | synthetic |
| Растворимость в воде | H2O: miscible alcohol: miscible diethyl ether: miscible |
| Мерк | 14,8021 |
| Номер JECFA | 936 |
| БРН | 506211 |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents, strong bases. Refrigerate. |
| ИнЧИКей | LCTONWCANYUPML-UHFFFAOYSA-N |
| LogP | -1.24 |
| Dissociation constant | 2.49 at 25℃ |
| Вещества, добавляемые в пищу (ранее EAFUS) | PYRUVIC ACID |
| FDA 21 CFR | 172.515 |
| Справочник по базе данных CAS | 127-17-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 8558G7RUTR |
| Справочник по химии NIST | Pyruvic acid(127-17-3) |
| Система регистрации веществ EPA | Propanoic acid, 2-oxo- (127-17-3) |
| UNSPSC Code | 12352106 |
| NACRES | NA.22 |
| Коды опасности | C | |||||||||
| Заявления о рисках | 34 | |||||||||
| Заявления о безопасности | 26-36/37/39-45-25-27 | |||||||||
| РИДАДР | UN 3265 8/PG 2 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | UZ0829800 | |||||||||
| Температура самовоспламенения | 305 °C | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 29335995 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H314:При попадании на кожу и в глаза вызывает химические ожоги.
-
оператор предупредительных мер
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P330+P331:ПРИ ПРОГЛАТЫВАНИИ: Прополоскать рот. Не вызывать рвоту!
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
ПировиноGRадная кислота химические свойства, назначение, производство
Химические свойства
Pyruvic acid has a sour, acetic odor (similar to acetic acid). It has a pleasant, sour taste with a burning, somewhat sweet note. It tends to darken and decompose unless kept free of minor contaminants and in tightly sealed containersВхождение
Isolated from cane sugar fermentation broth and from a few plants; also reported found in peppermint, raw asparagus, leaves and stalk of celery, onion, rutabaga, milk, cream, buttermilk, wheaten bread, blue cheeses, cheddar cheese, cottage cheese, provolone cheese, yogurt, beef, Virginia tobacco, beer, white wine, botrytised wine, cocoa and sake.Использование
pyruvic acid is an alpha hydroxy acid that can be irritating and is considered difficult to work with. It has a larger molecular size than the most commonly used AHAs. Sodium pyruvate is more commonly used, and is an organic salt.Подготовка
By distillation of tartaric acid in the presence of potassium acid sulfate as a dehydrating agent; from acetyl chloride and potassium cyanide to yield the nitrile, which is subsequently acid hydrolyzed to the acid; pyruvic acid must be rectified under vacuum.Определение
Pyruvic acid is an important organic chemical intermediate, an intermediate compound in the metabolism of carbohydrates, proteins, and fats, and is used in a variety of fields, including the pharmaceutical, cosmetic, food, and chemical industries. It has roles as a basic metabolite and cofactor. In thiamine deficiency, its oxidation is retarded and it tends to accumulate in neurostructural tissues causing neurological damage. In vitro studies have shown that pyruvic acid modulates cardiac function at physiological or low doses, but can cause cardiomyocyte damage at high doses.Общее описание
Pyruvic acid is the key component formed during the hydrolysis of flavor-precursors called S-alk(en)yl-L-cysteine-sulfoxides in onion tissues by allinase during maceration or chopping. The amount of pyruvic acid formed is used as a measure for onion pungency.Приложение биотехнологий?
Pyruvic acid is a key position in cell metabolism and is involved in many catabolic and anabolic pathways, including glycolysis, gluconeogenesis, amino acid, and protein metabolism. Pyruvic acid is employed for the production of L-tryptophan, L-tyrosine, and 3,4-dihydroxyphenyl alanine in various industries. The diet supplementation with pyruvic acid increased fat loss and minimized the associated loss of body protein. Pyruvic acid is also used in biochemical researches and medicine as a substrate for assaying activities of such enzymes as pyruvate dehydrogenase, pyruvate carboxylase, and pyruvate decarboxylase (Nakazawa et al. 1972; Yamada et al. 1972; Stanko et al. 1992).Y. lipolytica oxidize glucose and form pyruvic acid (75–80 %) and a-ketoglutaric acid (20–25 %) under thiamine deficiency conditions. The synthesis of the acid was triggered by a decrease in intracellular thiamine concentration to 3.0 lg per 1 g biomass. An approximately 3-fold increase in the amount of the biomass was associated with a subsequent decrease in thiamine content to the level of 1.0 lg per 1 g biomass, whose maximum production of pyruvic acid was 50 g/L in this condition. In addition to glucose, thiamine-auxotrophic yeasts are capable of synthesizing pyruvic acid when grown on glycerol and propionic acid. Technicalgrade glycerol is the most promising raw material for pyruvic acid production. Pyruvic acid was obtained at a concentration of 61 g/L with a yield of 71 % from glycerol (Morgunov et al. 2004; Finogenova et al. 2005).
Синтез
Pyruvic acid's synthesis method is as follows: from Tartaric acid by heating with hydrophilic agents, such as Potassium hydrosulfate.Методы очистки
Distil it twice, then fractionally crystallise it by partial freezing. [Beilstein 3 IV 1505.]ПировиноGRадная кислота запасные части и сырье
ПировиноGRадная кислота поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-852-30606658 | China | 43340 | 58 | ||
| +86-21-57256005 +86-15921811235 |
China | 20 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-86-4001020630 +8619831957301 |
China | 1000 | 58 | ||
| +8617732866630 | China | 18147 | 58 | ||
| +86-0533-2185556 +8617865335152 |
China | 10986 | 58 | ||
| +8619931165850 | China | 1000 | 58 | ||
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 |
ПировиноGRадная кислота Обзор)
1of4