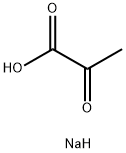
Натрий пируват
- английское имяSodium pyruvate
- CAS №113-24-6
- CBNumberCB4252757
- ФормулаC3H5NaO3
- мольный вес112.06
- EINECS204-024-4
- номер MDLMFCD00002586
- файл Mol113-24-6.mol
химическое свойство
| Температура плавления | >300 °C (lit.) |
| Температура кипения | >300℃ |
| плотность | 1,267g/cm |
| давление пара | 0Pa at 20℃ |
| показатель преломления | 1,426-1,43 |
| температура хранения | 2-8°C |
| растворимость | H2O: 100 mg/mL |
| форма | solution |
| цвет | White to Pale Yellow |
| РН | pH(100g/l, 25℃) : 4.0~8.0 |
| Биологические источники | synthetic (organic) |
| Растворимость в воде | Soluble in water. |
| БРН | 3568341 |
| Стабильность | Stable. Incompatible with strong oxidizing agents. |
| ИнЧИКей | DAEPDZWVDSPTHF-UHFFFAOYSA-M |
| LogP | -3.83 at 20℃ |
| Справочник по базе данных CAS | 113-24-6(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | POD38AIF08 |
| Система регистрации веществ EPA | Sodium pyruvate (113-24-6) |
| UNSPSC Code | 41116107 |
| NACRES | NA.75 |
больше
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/38-36/37/38 | |||||||||
| Заявления о безопасности | 37/39-26-24/25-36 | |||||||||
| WGK Германия | 3 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29183000 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H317:При контакте с кожей может вызывать аллергическую реакцию.
-
оператор предупредительных мер
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Натрий пируват химические свойства, назначение, производство
Описание
Sodium pyruvate is the sodium salt of pyruvate. It is frequently supplemented to the cell culture medium to act as a source of energy since it is a key intermediate during the production of the high-energy ATP molecules inside cells. For example, it can be used as a carbon source for bacteria. It may also protect cell against hydrogen peroxide, an oxidant to scavenger oxygen radicals. It is an important metabolic intermediate in many essential metabolic pathways such as carbohydrate metabolism. For example, it is converted into acetyl coenzyme A and enters into the TCA cycle (Kreb’s cycle) in organisms. It is also involved in the amino acid metabolism in organisms.Химические свойства
White to slightly yellow crystalline powder. soluble in water, very slightly soluble in ethanol.Использование
Sodium pyruvate is used in cell culture media as an additional source of energy. It acts as an antioxidant and finds protective effects against oxygen radicals. It serves as an intermediate in many metabolic pathways such as sugar metabolism. It is converted into acetyl coenzyme A and enters the trichloroacetic acid cycle. In addition, it is involved with amino acid metabolism and initiates the Kreb's cycle. It plays an important role as a free radical scavenger.прикладной
Sodium pyruvate has been used:To prepare Bull Tyrode′s albumin-lactate-pyruvate diluent.
In pyruvate tolerance test.
To prepare oligodendrocyte culture medium.
Подготовка
The preparation of sodium pyruvate is as follows:Synthesis of pyruvate, a salt of a compound of formula (II) by ozonolysis of methacrylic acid, a compound of formula (II) A solution of methacrylic acid obtained in Example la or lb (15.31 g, 178 mmol) in dichloromethane/methanol (5 % methanol, 50 ml) was cooled to -78 0C and a stream of ozone was passed through until the solution turned blue. Dimethylsulfide (12.41 g, 200 mmol) was added and the mixture was allowed to reach room temperature. Excess dimethylsulfide was removed by passing a stream of nitrogen through the reaction mixture. The reaction mixture was evaporated in vacuo at 30 0C. To the residue was added water (100 ml) and then slowly added a solution of aq. NaOH (IM, 178 ml). The mixture was concentrated and left for crystallisation at 4 °C. The white crystalline material obtained was filtered and washed with acetone (3x100 ml). Yield: 14.50 g (92 %). Purity: 95 %.Биологические функции
Sodium pyruvate (α-Ketopropionic acid sodium salt; 2-Oxopropanoic acid sodium salt;Pyruvic acid sodium; C3H3NaO3) as an important endogenous small molecules participates various tissue and organ metabolism processes that is the final product of glycolysis and the starting substrate for the tricarboxylic acid (TCA) cycle, and possesses antioxidant and scavenging free radical effects, thus widely using as buffer, excipient and antioxidant in medicine, diagnostic reagent and medical device.Sodium pyruvate is an endogenous antioxidant and reactive oxygen radical scavenger. In this process, H2O2 or other reactive oxygen radicals are scavenged by sodium pyruvate by a nonenzymatic reaction or an oxidative dephosphorylation, and produce acetate, water and carbon dioxide. Thus, sodium pyruvate can suppress renal cellular injury induced by H2O2, such as lipid peroxidation of rat kidney homogenate, and cytosolic 51Cr release (a marker of cellular injury) from renal epithelial cells induced by H2O2. Thus, sodium pyruvate as effect antioxidant has potential in the clinical medication. Moreover, as effect antioxidant, sodium pyruvate is also as additives used in a vast range of foods and toiletries.
Общее описание
Sodium pyruvate (SP) is a simplest keto-acid. It crystallizes as very thin plates and belongs to the monoclinic system. Its utility in Knoevenagel condensation carried out in an aqueous medium has been examined. The standard molar enthalpy of formation and molar enthalpy of dissolution of SP at infinite dilution have been obtained. Its efficacy in treating mitochondrial DNA depletion syndromes has been investigated.хранилище
Sterile filtered commercial solutions of sodium pyruvate are stable up to 24 months, when stored at 2-8 °C.Pyruvic acid polymerizes and decomposes upon standing. It is advised to keep containers tightly sealed.
использованная литература
https://en.wikipedia.org/wiki/Sodium_pyruvateTaidi, Behnam, et al. "Effect of carbon source and concentration on the molecular mass of poly (3-hydroxybutyrate) produced by Methylobacterium extorquens and Alcaligenes eutrophus." Applied microbiology and biotechnology 40.6 (1994): 786-790.
https://www.alfa.com/zh-cn/catalog/A11148/
http://bio.lonza.com/uploads/tx_mwaxmarketingmaterial/Lonza_BenchGuides_Sodium_Pyruvate_Solution_100mM.pdf
Натрий пируват запасные части и сырье
сырьё
запасной предмет
1of3
Натрий пируват поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-21-57256005 +86-15921811235 |
China | 20 | 58 | ||
| +8613343047651 | China | 3692 | 58 | ||
| +8613393234347 | China | 2946 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +16837774622 | China | 295 | 58 | ||
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | ||
| +8618092446649 | China | 1143 | 58 | ||
| +8619933239880 | China | 861 | 58 |
Натрий пируват Обзор)
1of4