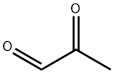
ПировиноGRадная альдегид
- английское имяMethylglyoxal
- CAS №78-98-8
- CBNumberCB1157969
- ФормулаC3H4O2
- мольный вес72.06
- EINECS201-164-8
- номер MDLMFCD00006960
- файл Mol78-98-8.mol
| Температура плавления | 25 °C |
| Температура кипения | 72 °C |
| плотность | 1.19 g/mL at 20 °C |
| давление пара | 25.09hPa at 20℃ |
| показатель преломления | n |
| FEMA | 2969 | PYRUVALDEHYDE |
| RTECS | UZ0700000 |
| температура хранения | 2-8°C |
| растворимость | DMSO (Slightly), Methanol (Slightly), Water (Soluble) |
| форма | Solution |
| цвет | Clear yellow to yellow-brown |
| Запах | at 1.00 % in propylene glycol. sweet acidic ethereal brown rum |
| Odor Type | caramellic |
| Биологические источники | synthetic |
| Растворимость в воде | >=10 g/100 mL at 17 ºC |
| Чувствительный | Air Sensitive |
| Мерк | 14,6081 |
| Номер JECFA | 937 |
| БРН | 906750 |
| ИнЧИКей | AIJULSRZWUXGPQ-UHFFFAOYSA-N |
| LogP | -1.06 at 25℃ |
| Вещества, добавляемые в пищу (ранее EAFUS) | PYRUVALDEHYDE |
| FDA 21 CFR | 172.515 |
| Справочник по базе данных CAS | 78-98-8(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 722KLD7415 |
| МАИР | 3 (Vol. 51) 1991 |
| Справочник по химии NIST | Propanal, 2-oxo-(78-98-8) |
| Система регистрации веществ EPA | Methylglyoxal (78-98-8) |
| UNSPSC Code | 12352204 |
| NACRES | NA.32 |
| Коды опасности | Xn,Xi,C | |||||||||
| Заявления о рисках | 22-36-35 | |||||||||
| Заявления о безопасности | 26-36-45-36/37/39 | |||||||||
| РИДАДР | UN 3265 8 / PGIII | |||||||||
| WGK Германия | - | |||||||||
| Примечание об опасности | Irritant | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29121900 | |||||||||
| Банк данных об опасных веществах | 78-98-8(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H318:При попадании в глаза вызывает необратимые последствия.
H317:При контакте с кожей может вызывать аллергическую реакцию.
H341:Предполагается, что данное вещество вызывает генетические дефекты.
H290:Может вызывать коррозию металлов.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P234:Хранить только в оригинальной упаковке.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
ПировиноGRадная альдегид химические свойства, назначение, производство
Описание
Methylglyoxal (MG) is a highly reactive a-dicarbonyl compound that is primarily generated endogenously during glycolytic pathways (glucose and fructose metabolism) in cells and exogenously due to autoxidation of sugar, degradation of lipids, and fermentation during food and drink processing. Methylglyoxal polymerizes readily; it is hygroscopic and incompatible with strong oxidizing agents and bases. Methylglyoxal may be present as a free molecule in the diet or bound to biological materials, such as proteins, and as advanced glycation end products (AGEs), which are poorly absorbed. Methylglyoxal has been indicated in pathological events associated with hyperglycemia in both type 1 and type 2 diabetes and in other diabetic complications as either a direct toxin or as a precursor for AGEs. In animal studies, MG has been shown to induce tumorigenesis, but has also been reported as a tumoristatic agent. Methylglyoxal has been identified as the dominant antibacterial constituent of manuka honey.Химические свойства
clear yellow to yellow-brown solutionВхождение
Reported found in the dry distillate of Manilla copal. Also reported found in apple juice, orange juice, celery root, rutabaga, tomato, wheaten bread, white bread, roasted and raw turkey, cognac, roasted barley, beer, cocoa, coffee and roasted pecans.Использование
Methylglyoxal solution has been used:- to assess glyoxalase 1 (GLO1) enzymatic activity
- as an advanced glycation end (AGE) forming agent for the preparation of albumin in vitro
- to regulate anxiety like behavior in mice
- to induce peritoneal fibrosis in rats
- to study the chromatographic retention characteristics of organic chemicals and metal DNA adducts
- for intraplantar injection in mice to investigate peripheral and central components of methylglyoxal (MG)-transient receptor potential ankyrin 1 (TRPA1)-adenylyl cyclase 1 isoform (AC1) pathway
Подготовка
By distilling a dilute solution of dihydroxyacetone from calcium carbonate; by oxidation of acetone with selenium dioxide; by heating dihydroxy acetone with phosphorus pentoxide; by warming isonitroso acetone with diluted H2SO4.Определение
ChEBI: A 2-oxo aldehyde derived from propanal.Общее описание
Clear yellow slightly viscous liquid with a pungent odor. Yellowish-green vapors. Faintly acidic to litmus.Реакции воздуха и воды
Water soluble.Профиль реактивности
Methylglyoxal polymerizes readily. Methylglyoxal is hygroscopic. Methylglyoxal is incompatible with strong oxidizing agents and bases. Methylglyoxal is an aldehyde. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation.Пожароопасность
Literature sources indicate that Methylglyoxal is nonflammable.Экологическая судьба
Methylglyoxal production and use as a chemical intermediate and flavoring agent may result in its release to the environment through various waste streams. If released into water, MG is not expected to adsorb to suspended solids and sediment based on the estimated Koc. Volatilization from water surfaces is not expected to be an important fate process based upon the estimated Henry’s Law constant. If released to soil, MG is expected to have very high mobility based upon an estimated Koc of 1 determined from the structure estimation method. Hydrolysis is not expected to be an important environmental fate process since this compound lacks functional groups that hydrolyze under environmental conditions.Methylglyoxal serves as a substrate for the isozymes E1, E2, and E3 of human aldehyde dehydrogenase. Oxidation of MG by these isozymes generated pyruvate. Methylglyoxal is a partially oxidized compound obtained from the tropospheric oxidation of numerous hydrocarbons, of both biogenic and anthropogenic origin. If released to the air, an estimated vapor pressure of 27 mm Hg at 25 ℃ indicates MG will exist solely as a vapor in the atmosphere. Vapor-phase MG will be degraded in the atmosphere by reaction with photochemically produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 30 h. Methylglyoxal absorbs light at wavelengths >290 nm and, therefore, is susceptible to direct photolysis by sunlight; half-lives of 2–4 h have been reported.
Методы очистки
Commercial 30% (w/v) aqueous solution is diluted to about 10% and distilled twice, taking the fraction boiling below 50o/20mm Hg. (This treatment does not remove lactic acid). [Beilstein 1 IV 3631.]ПировиноGRадная альдегид запасные части и сырье
ПировиноGRадная альдегид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-0311-87836622 +86-17333973358 |
China | 8051 | 58 | |
| +8617732866630 | China | 18147 | 58 | |
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 |