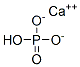
Кальция гидрофосфат
- английское имяCalcium phosphate dibasic
- CAS №7757-93-9
- CBNumberCB6344120
- ФормулаCaHO4P
- мольный вес136.06
- EINECS231-826-1
- номер MDLMFCD00010909
- файл Mol7757-93-9.mol
химическое свойство
| Температура плавления | 370°C(decomposition) |
| плотность | 2.306(16℃) |
| RTECS | TB8528000 |
| температура хранения | 2-8°C |
| растворимость | Practically insoluble in water and in ethanol (96 per cent). It dissolves in dilute hydrochloric acid and in dilute nitric acid. |
| форма | gel (aged) |
| цвет | White |
| РН | 7 (10g/l, H2O, 20°C) suspension |
| Растворимость в воде | Sparingly soluble in water, practically insoluble in cold water. |
| Мерк | 13,1697 |
| Константа произведения растворимости (Ksp) | pKsp: 7 |
| Стабильность | Stable. Incompatible with strong oxidizing agents. |
| ИнЧИКей | FUFJGUQYACFECW-UHFFFAOYSA-L |
| LogP | -2.148 (est) |
| Вещества, добавляемые в пищу (ранее EAFUS) | CALCIUM PHOSPHATE, DIBASIC |
| Специальный комитет по веществам GRAS | Calcium phosphate dibasic |
| FDA 21 CFR | 181.29 |
| Справочник по базе данных CAS | 7757-93-9(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | L11K75P92J |
| Система регистрации веществ EPA | Calcium hydrogen phosphate (7757-93-9) |
| UNSPSC Code | 12352302 |
| NACRES | NA.25 |
больше
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/37/38 | |||||||||
| Заявления о безопасности | 26-36-24/25-22 | |||||||||
| WGK Германия | 3 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2835 25 00 | |||||||||
| Банк данных об опасных веществах | 7757-93-9(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 10000 mg/kg LD50 dermal Rabbit > 7940 mg/kg | |||||||||
| NFPA 704: |
|
Кальция гидрофосфат химические свойства, назначение, производство
Химические свойства
Anhydrous dibasic calcium phosphate is a white, odorless, tasteless powder or crystalline solid. It occurs as triclinic crystals.Физические свойства
White triclinic crystal; density 2.92 g/cm3 (anhydrous) and 2.31 g/cm3 (dihy drate); hardness 3.5 Mohs; decomposes on heating; inosluble in water and alcohol; KSP 2.7x10-7; soluble in dilute mineral acid.Использование
Replenisher (calcium); pharmaceutic aid (tablet base).Методы производства
Calcium phosphates are usually prepared by reacting very pure phosphoric acid with calcium hydroxide, Ca(OH)2 obtained from limestone, in stoichiometric ratio in aqueous suspension followed by drying at a temperature that will allow the correct hydration state to be achieved. After drying, the coarse-grade material is obtained by means of a classification unit; the fine particle-size material is obtained by milling. Dibasic calcium phosphate, anhydrous, may also be prepared by spray-drying.Сельскохозяйственное использование
Dicalcium phosphate (CaHPO4) is made from calcium carbonate and phosphoric acid. It contains 34% citrate- soluble phosphorus pentoxide (P2O5). It is not commonly used as a fertilizer, but is used as a supplement to animal feed.Фармацевтические приложения
Anhydrous dibasic calcium phosphate is used both as an excipient and as a source of calcium in nutritional supplements. It is used particularly in the nutritional/health food sectors. It is also used in pharmaceutical products because of its compaction properties, and the good flow properties of the coarse-grade material. The predominant deformation mechanism of anhydrous dibasic calcium phosphate coarse-grade is brittle fracture and this reduces the strain-rate sensitivity of the material, thus allowing easier transition from the laboratory to production scale. However, unlike the dihydrate, anhydrous dibasic calcium phosphate when compacted at higher pressures can exhibit lamination and capping. This phenomenon can be observed when the material represents a substantial proportion of the formulation, and is exacerbated by the use of deep concave tooling. This phenomenon also appears to be independent of rate of compaction.Anhydrous dibasic calcium phosphate is abrasive and a lubricant is required for tableting, for example 1% w/w magnesium stearate or 1% w/w sodium stearyl fumarate.
Two particle-size grades of anhydrous dibasic calcium phosphate are used in the pharmaceutical industry. Milled material is typically used in wet-granulated or roller-compacted formulations. The ‘unmilled’ or coarse-grade material is typically used in directcompression formulations.
Anhydrous dibasic calcium phosphate is nonhygroscopic and stable at room temperature. It does not hydrate to form the dihydrate.
Anhydrous dibasic calcium phosphate is used in toothpaste and dentifrice formulations for its abrasive properties.
Профиль безопасности
Skin and eye irritant. A nuisance dust.Безопасность
Dibasic calcium phosphate anhydrous is widely used in oral pharmaceutical products, food products, and toothpastes, and is generally regarded as a relatively nontoxic and nonirritant material.хранилище
Dibasic calcium phosphate anhydrous is a nonhygroscopic, relatively stable material. Under conditions of high humidity it does not hydrate to form the dihydrate.The bulk material should be stored in a well-closed container in a dry place.
Методы очистки
Crystallise it from a near-saturated solution in 50% aqueous reagent grade phosphoric acid at 100o by filtering through fritted glass and cooling to room temperature. The crystals are filtered off, and this process is repeated three times using fresh acid. For the final crystallisation the solution is cooled slowly with constant stirring to give thin plate crystals that are filtered off on a fritted glass funnel, washed free of acid with anhydrous acetone and dry in a vacuum desiccator [Egan et al.J Am Chem Soc 78 1811 1956].Несовместимости
Dibasic calcium phosphate should not be used to formulate tetracyline antibiotics.The surface of milled anhydrous dibasic calcium phosphate is alkaline and consequently it should not be used with drugs that are sensitive to alkaline pH. However, reports suggest there are differences in the surface alkalinity/acidity between the milled and unmilled grades of anhydrous dibasic calcium phosphate; the unmilled form has an acidic surface environment. This difference has important implications for drug stability, particularly when reformulating from, e.g. roller compaction to direct compression, when the particle size of the anhydrous dibasic calcium phosphate might be expected to change.
Dibasic calcium phosphate dihydrate has been reported to be incompatible with a number of drugs and excipients, and many of these incompatibilities are expected to occur with dibasic calcium phosphate, anhydrous; see Calcium phosphate, dibasic dihydrate.
Регуляторный статус
GRAS listed. Accepted as a food additive in Europe. Included in the FDA Inactive Ingredients Database (oral capsules and tablets). Included in nonparenteral medicines licensed in Europe. Included in the Canadian List of Acceptable Non-medicinal Ingredients.Кальция гидрофосфат запасные части и сырье
сырьё
- Кальция фосфат, моноосновной
- Phosphate rock powder
- Метиловый красный
- Calcium chloride solution 36-40%, (1box=27kgs)
- CALCIUM CARBONATE
- альция оксид
- PHOS ROCK FERTILIZER IN BULK
- Calcium phosphate monobasic
- calcium magnesium phosphate(CMP)
- Хлорид натрия
1of4
Кальция гидрофосфат поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-13637972665 +86-13637972665 |
China | 81 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8617732866630 | China | 18147 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | ||
| +8613031735486 | China | 105 | 58 | ||
| +86-551-62622640 | China | 293 | 58 | ||
| +8618092446649 | China | 1143 | 58 | ||
| +86-86-17798073498 +8617798073498 |
China | 299 | 58 |