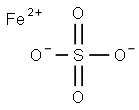
Железа сульфат
- английское имяFERROUS SULFATE
- CAS №7720-78-7
- CBNumberCB7450570
- ФормулаFeO4S
- мольный вес151.91
- EINECS231-753-5
- номер MDLMFCD00011014
- файл Mol7720-78-7.mol
| Температура плавления | decomposes at 671℃ [JAN85] |
| плотность | 3.650 |
| давление пара | 0Pa at 20℃ |
| температура хранения | Store at +15°C to +25°C. |
| растворимость | Water (Slightly) |
| форма | white orthorhombic crystals |
| цвет | white orthorhombic crystals, crystalline; hygroscopic |
| Запах | at 100.00?%. odorless |
| РН | 2.5 (20°C, 50g/L in H2O) |
| Растворимость в воде | g/100g solution H2O: 13.6 (0°C), 22.8 (25°C), 24.0 (100°C); solid phase, FeSO4 · 7H2O (0°C, 25°C), FeSO4 ·H2O (100°C) [KRU93] |
| Диэлектрическая постоянная | 14.2(14℃) |
| InChI | InChI=1S/Fe.H2O4S/c;1-5(2,3)4/h;(H2,1,2,3,4)/q+2;/p-2 |
| ИнЧИКей | BAUYGSIQEAFULO-UHFFFAOYSA-L |
| SMILES | [Fe+2].S([O-])([O-])(=O)=O |
| FDA 21 CFR | 184.1315; 582.5315; 310.545; 582.80 |
| Специальный комитет по веществам GRAS | Ferrous sulfate Ferrous sulfate (packaging) |
| Справочник по базе данных CAS | 7720-78-7(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1-5 |
| FDA UNII | 2IDP3X9OUD |
| Словарь онкологических терминов NCI | ferrous sulfate |
| Словарь наркотиков NCI | ferrous sulfate |
| Код УВД | B03AA07,B03AD03 |
| Справочник по химии NIST | Ferrous sulfate(7720-78-7) |
| Система регистрации веществ EPA | Ferrous sulfate (7720-78-7) |
| UNSPSC Code | 12352103 |
| NACRES | NA.21 |
| Заявления о рисках | 25 |
| Заявления о безопасности | 45 |
| кода HS | 28332910 |
| Банк данных об опасных веществах | 7720-78-7(Hazardous Substances Data) |
| Токсичность | LD50 oral in rat: 319mg/kg |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P332+P313:При возникновении раздражения кожи: обратиться за медицинской помощью.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Железа сульфат химические свойства, назначение, производство
Описание
Green vitriol, FeSO4.7H20, has been known since the thirteenth century ; it crystallizes from solutions of iron or iron bases in dilute sulphuric acid. The heptahydrate forms green monoclinic crystals of density 1·88, very soluble in water (296 g litre-1 FeS04 at 25°C). By precipitating the aqueous solution with ethanol, heating the heptahydrate to 140° in vacuo or by crystallizing it from 50 % sulphuric acid, the white monohydrate is obtained. This can be further dehydrated to the white, amorphous FeSO4 by heating to 300° in a current of hydrogen. At red heat the sulphate decomposes : 2FeS04 -> Fe203+S02+S03 A tetrahydrate, FeS04.4H20, crystallizes from aqueous solutions above 56°.Химические свойства
Ferrous sulfate is a greenish or yellowish solid in fine or lumpy crystals.Физические свойства
White orthorhombic crystal; hygroscopic; density 3.65 g/cm3; soluble in water (26.6g/100g water at 20°C). The monohydrate is a yellowish-white monoclinic crystal; density 3.0 g/cm3; decomposes at 300°C; soluble in water. Heptahydrate is bluish-green monoclinic crystal; refractive index 1.47; hardness 2 Mohs; density 1.89g/cm上3; decomposes at about 60°C; very soluble in water; soluble in absolute methanol; slightly soluble in ethanol.Вхождение
Iron(II) sulfate is probably the most important salt of iron, as well as the longest-known iron(II) compound. The compound is used as a mordant in dyeing; as a component of writing ink; in electroplating baths; in radiation dosimeters; in lithography and engraving; as a weed-killer; and in water purification. A major application of this compound is in the manufacture of other iron(II) salts including Prussian blue or ferric ferrocyanide. Iron(II) sulfate also is used as a reducing agent and an analytical reagent (in brown ring test for nitrate).Использование
Ferrous Sulfate is a nutrient and dietary supplement that is a source of iron. it is a white to grayish odorless powder. ferrous sulfate hep- tahydrate contains approximately 20% iron, while ferrous sulfate dried contains approximately 32% iron. it dissolves slowly in water and has high bioavailability. it can cause discoloration and rancidity. it is used for fortification of baking mixes. in the encapsulated form it does not react with lipids in cereal flours. it is used in infant foods, cereals, and pasta products.Методы производства
Iron(II) sulfate in industrial scale is mostly produced in the pickling process as a by-product of the steel industry. It is obtained when the surface of steel is cleaned with dilute sulfuric acid to remove metal impurities. In the laboratory iron(II) sulfate heptahydrate may be prepared by dissolving iron in dilute sulfuric acid in a reducing atmosphere, followed by crystallization:Fe + H2SO4 → FeSO4 + H2
Alcohol may be added to the aqueous solution to speed up crystallization; iron(II) may otherwise oxidize to iron(III) during a slow crystallization process.
Iron(II) oxide or carbonate may be used instead of iron metal to prepare the heptahydrate.
.
Определение
A rusty-brown solid prepared by the action of heat on iron(III) hydroxide or iron(II) sulfate. It occurs in nature as the mineral hematite. Industrially it is obtained by roasting iron pyrites. Iron(III) oxide dissolves in dilute acids to produce solutions of iron(III) salts. It is stable at red heat, decomposes around 1300°C to give triiron tetroxide, and can be reduced to iron by hydrogen at 1000°C. Iron(III) oxide is not ionic in character but has a structure similar to that of aluminum(III) oxide.Опасность
Ingestion causes intestinal disorders.Сельскохозяйственное использование
Copperas, also called green vitriol, is ferrous sulphate heptahydrate. It is an iron salt fertilizer, which is most effective in overcoming iron deficiency.Профиль безопасности
A human poison by ingestion. Moderately toxic to humans by an unspecified route. An experimental poison by ingestion, intraduodenal, intraperitoneal, intravenous, and subcutaneous routes. Human systemic effects by ingestion: aggression, somnolence, brain recorlng changes, diarrhea, nausea or vomiting, bleedmg from the stomach, coma. Questionable carcinogen with experimental tumorigenic data. Experimental teratogenic and reproductive effects. Mutation data reported. Potentially explosive reaction with methyl isocyanoacetate at 25'. May igmte on contact with arsenic trioxide + sodium nitrate. When heated to decomposition it emits toxic fumes of SOx. See also IRON COMPOUNDS.Возможный контакт
It is used as a fertilizer, food or feed additive; and in herbicides; process engraving; dyeing, and water treatment. A byproduct of various chemical and metal treating operations.Несовместимости
Aqueous solution is acidic. Contact with alkalies form iron. Keep away from alkalies, soluble carbo nates; gold and silver salts; lead acetate; lime water, potassium iodide; potassium and sodium tartrate; sodium borate; tannin.Железа сульфат запасные части и сырье
сырьё
1of2
запасной предмет
- Iron Oxide Green
- High temperature shift calalyst
- Naphthenate drier
- анамицина сульфа
- Гентамицина сульфа
- Iron oxide
- Foliar-fertilizer
- Desulfurizer
- Железо (III) гексацианоферрат (II)
- Ammonium iron(II) sulfate
1of4
Железа сульфат поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8618805133257 | China | 132 | 60 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8617798174412 | China | 2097 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-17736087130 +86-18633844644 |
China | 994 | 58 | ||
| +86-86-17798073498 +8617798073498 |
China | 299 | 58 |