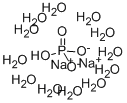
Sodium phosphate dibasic dodecahydrate BioXtra
- английское имяSodium phosphate dibasic dodecahydrate
- CAS №10039-32-4
- CBNumberCB1324215
- ФормулаH25Na2O16P
- мольный вес358.14
- EINECS600-088-6
- номер MDLMFCD00149181
- файл Mol10039-32-4.mol
химическое свойство
| Температура плавления | 35 °C |
| Плотность накопления | 800-900kg/m3 |
| плотность | 1,52 g/cm3 |
| температура хранения | Store at +15°C to +25°C. |
| растворимость | H2O: 0.1 M at 20 °C, clear, colorless |
| форма | Crystals |
| цвет | White |
| РН | 9.0-9.3 (25℃, 50mg/mL in H2O) |
| Запах | Odorless |
| Водородный показатель | 8.4 - 9.6 at 36 g/l at 25 °C |
| Растворимость в воде | 218 g/L (20 ºC) |
| λмакс | λ: 260 nm Amax: 0.01 λ: 280 nm Amax: 0.01 |
| InChI | InChI=1S/2Na.H3O4P.12H2O/c;;1-5(2,3)4;;;;;;;;;;;;/h;;(H3,1,2,3,4);12*1H2/q2*+1;;;;;;;;;;;;;/p-2 |
| ИнЧИКей | DGLRDKLJZLEJCY-UHFFFAOYSA-L |
| SMILES | P([O-])([O-])(=O)O.O.O.O.O.O.O.O.O.O.O.O.O.[Na+].[Na+] |
| FDA 21 CFR | 175.210; 175.300; 181.29 |
| Справочник по базе данных CAS | 10039-32-4(CAS DataBase Reference) |
| FDA UNII | E1W4N241FO |
| Система регистрации веществ EPA | Disodium phosphate dodecahydrate (10039-32-4) |
| UNSPSC Code | 12352302 |
| NACRES | NA.25 |
больше
| Заявления о безопасности | 24/25 |
| РИДАДР | UN 3077 9 / PGIII |
| WGK Германия | 1 |
| RTECS | TC5725000 |
| кода HS | 28352200 |
| Токсичность | LD50 orally in Rabbit: > 2000 mg/kg |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
-
оператор предупредительных мер
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Sodium phosphate dibasic dodecahydrate BioXtra химические свойства, назначение, производство
Химические свойства
white crystalsИспользование
Sodium Phosphate Dibasic Dodecahydrate is used in method for preparing self-supporting membrane.Методы производства
Either bone phosphate (bone ash), obtained by heating bones to whiteness, or the mineral phosphorite is used as a source of tribasic calcium phosphate, which is the starting material in the industrial production of dibasic sodium phosphate.Tribasic calcium phosphate is finely ground and digested with sulfuric acid. This mixture is then leached with hot water and neutralized with sodium carbonate, and dibasic sodium phosphate is crystallized from the filtrate.
Определение
ChEBI: A hydrate that is the dodecahydrate form of disodium hydrogenphosphateОбщее описание
Sodium phosphate is a colorless crystalline sodium salt. It is widely used as a buffer in molecular biology, biochemistry, and chromatographic studies. It exists in three forms: monobasic (NaH2PO4), dibasic (Na2HPO4), and tribasic (Na3PO4). Monobasic and dibasic forms in varying ratios are commonly used to compose the neutral sodium phosphate buffer solution.Фармацевтические приложения
Dibasic sodium phosphate is used in a wide variety of pharmaceutical formulations as a buffering agent and as a sequestering agent. Therapeutically, dibasic sodium phosphate is used as a mild laxative and in the treatment of hypophosphatemia.Dibasic sodium phosphate is also used in food products; for example as an emulsifier in processed cheese.
Безопасность
Dibasic sodium phosphate is widely used as an excipient in parenteral, oral, and topical pharmaceutical formulations. Phosphate occurs extensively in the body and is involved in many physiological processes since it is the principal anion of intracellular fluid. Most foods contain adequate amounts of phosphate, making hypophosphatemia (phosphate deficiency) virtually unknown except for certain disease states or in patients receiving total parenteral nutrition. Treatment is usually by the oral administration of up to 100 mmol of phosphate daily.Approximately two-thirds of ingested phosphate is absorbed from the gastrointestinal tract, virtually all of it being excreted in the urine, and the remainder is excreted in the feces.
Excessive administration of phosphate, particularly intravenously, rectally, or in patients with renal failure, can cause hyperphosphatemia that may lead to hypocalcemia or other severe electrolyte imbalances. Adverse effects occur less frequently following oral consumption, although phosphates act as mild saline laxatives when administered orally or rectally. Consequently, gastrointestinal disturbances including diarrhea, nausea, and vomiting may occur following the use of dibasic sodium phosphate as an excipient in oral formulations. However, the level of dibasic sodium phosphate used as an excipient in a pharmaceutical formulation is not usually associated with adverse effects.
LD50 (rat, oral): 17 g/kg
хранилище
The anhydrous form of dibasic sodium phosphate is hygroscopic. When heated to 40℃, the dodecahydrate fuses; at 100℃ it loses its water of crystallization; and at a dull-red heat (about 240℃) it is converted into the pyrophosphate, Na4P2O7. Aqueous solutions of dibasic sodium phosphate are stable and may be sterilized by autoclaving.The bulk material should be stored in an airtight container, in a cool, dry place.
Несовместимости
Dibasic sodium phosphate is incompatible with alkaloids, antipyrine, chloral hydrate, lead acetate, pyrogallol, resorcinol and calcium gluconate, and ciprofloxacin. Interaction between calcium and phosphate, leading to the formation of insoluble calcium-phosphate precipitates, is possible in parenteral admixtures.Регуляторный статус
GRAS listed. Accepted in Europe for use as a food additive. Included in the FDA Inactive Ingredients Database (injections; infusions; nasal, ophthalmic, oral, otic, topical, and vaginal preparations). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.Sodium phosphate dibasic dodecahydrate BioXtra запасные части и сырье
Sodium phosphate dibasic dodecahydrate BioXtra поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-13637972665 +86-13637972665 |
China | 81 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-027-59207850 | China | 5972 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | ||
| +8618092446649 | China | 1143 | 58 | ||
| +86-18633929156 +86-18633929156 |
China | 972 | 58 | ||
| +86-86-17798073498 +8617798073498 |
China | 299 | 58 | ||
| +86-18532138899 +86-18532138899 |
China | 939 | 58 |
Sodium phosphate dibasic dodecahydrate BioXtra Обзор)
- ди-Натрий hydrogen фосфат дигидрат EP
- Этилендиаминтетрауксусной кислоты динатриевая сол
- Триэтилфосфат
- Кальций фосфорнокислый
- Диметил сукцина
- Sodium phosphate monobasic monohydrate
- Цинк фосфат (орто)
- Монофторфосфат натрия
- Фосфат натрия водорода
- Натрия сукцинат
1of4