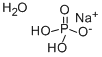
Sodium phosphate monobasic monohydrate
- английское имяSodium Phosphate Monobasic Monohydrate
- CAS №10049-21-5
- CBNumberCB5158267
- ФормулаH4NaO5P
- мольный вес137.992291
- EINECS600-102-0
- номер MDLMFCD00081857
- файл Mol10049-21-5.mol
| Температура плавления | 100°C -H₂O |
| Температура кипения | 399 °C |
| плотность | 2,04 g/cm3 |
| Плотность накопления | 880kg/m3 |
| температура хранения | Store at +5°C to +30°C. |
| растворимость | H2O: 1 M, clear, colorless |
| форма | Solid |
| пка | 7.21 |
| цвет | White semi-transparentor |
| РН | 4.1-4.5 (25℃, 50mg/mL in H2O) |
| Запах | Odorless |
| Водородный показатель | 4.1 - 4.5 at 50 g/l at 25 °C |
| Растворимость в воде | Soluble in water; insoluble in ethanol, ether and chloroform. |
| Чувствительный | Hygroscopic |
| λмакс | λ: 260 nm Amax: ≤0.03 λ: 280 nm Amax: ≤0.02 |
| Мерк | 14,8660 |
| ИнЧИКей | BBMHARZCALWXSL-UHFFFAOYSA-M |
| LogP | -2.148 (est) |
| Справочник по базе данных CAS | 10049-21-5(CAS DataBase Reference) |
| FDA 21 CFR | 310.545 |
| FDA UNII | 593YOG76RN |
| Система регистрации веществ EPA | Monosodium phosphate monohydrate (10049-21-5) |
| Поглощение | ≤0.01 at 260nm ≤0.01 at 280nm |
| UNSPSC Code | 41171610 |
| NACRES | NB.79 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/37/38-36 | |||||||||
| Заявления о безопасности | 26-36/37/39-36-39-24/25 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | WA1900000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2835 22 00 | |||||||||
| Токсичность | LD50 orally in Rabbit: > 2000 mg/kg LD50 dermal Rabbit > 7940 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P304+P340:ПРИ ВДЫХАНИИ: Свежий воздух, покой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P405:Хранить в недоступном для посторонних месте.
Sodium phosphate monobasic monohydrate химические свойства, назначение, производство
Описание
Sodium Phosphate Monobasic Monohydrate is a reagent with very high buffering capacity widely used in molecular biology, biochemistry and chromatography. it can be used as a buffer to adjust pH. In medicine, it is sometimes used as a stimulant laxative before certain operations and medical procedures. Sodium Phosphate Monobasic Monohydrate is often used in foods and in water treatment. It is used as sequestrant, emulsifier, mordant in dyeing, reagent and buffer in foods and analytical chemistry. It is applied as a fireproofing agent and for weighting silk in tanning. It is employed in manufacturing of enamels, ceramics, detergents, boiler compounds, in soldering and brazing instead of borax.Monobasic sodium phosphate is used in baking powders, acid cleansers, electroplating, as a dry acidulant, and in treating boiler water.
Химические свойства
Anhydrous salt: white crystalline powder; slightly hygroscopic; forms sodium acid pyrophosphate, Na2H2P2O7 on heating above 225°C and sodium metaphosphate (NaPO3)n at about 350 to 400°C; very soluble in water, aqueous solution acidic.Monohydrate: white orthorhombic crystals or granules; density 2.04 g/cm3 ; loses its water of crystallization at 100°C; very soluble in water, pH of 1% solution 4.5; insoluble in alcohol.
Dihydrate: large transparent crystals; orthorhombic bisphenoidal structure; density 1.915 g/cm 3 ; decomposes at 60°C; very soluble in water; insoluble in alcohol.
Использование
Sodium Phosphate Monobasic Monohydrate is often used in foods and in water treatment. it can be used as a buffer to adjust pH. In medicine, it is sometimes used as a stimulant laxative before certain operations and medical procedures.Методы производства
Monobasic sodium phosphate is prepared by adding phosphoric acid to a hot, concentrated solution of disodium phosphate until the liquid ceases to form a precipitate with barium chloride. This solution is then concentrated and the monobasic sodium phosphate is crystallized.Подготовка
Monobasic sodium phosphate can be prepared by partial neutralization of phosphoric acid with sodium hydroxide in equimolar amounts:H3PO4+ NaOH →NaH2PO4+ H2O
It also can be made by treating disodium hydrogen phosphate with phosphoric acid in proper stoichiometric amount:
Na2HPO4+ H3PO4→2NaH2PO4
Общее описание
Useful in conjuction with sodium phosphate, dibasic (Cat. No. 567550) in the preparation of biological buffers(absorbance: ≤0.01 at 260 nm and 280 nm).Фармацевтические приложения
Monobasic sodium phosphate is used in a wide variety of pharmaceutical formulations as a buffering agent and as a sequestering agent. Therapeutically, monobasic sodium phosphate is used as a mild saline laxative and in the treatment of hypophosphatemia.Monobasic sodium phosphate is also used in food products, for example, in baking powders, and as a dry acidulant and sequestrant.
Безопасность
Monobasic sodium phosphate is widely used as an excipient in parenteral, oral, and topical pharmaceutical formulations.Phosphate occurs extensively in the body and is involved in many physiological processes since it is the principal anion of intracellular fluid. Most foods contain adequate amounts of phosphate, making hypophosphatemia virtually unknown except in certain disease states or in patients receiving total parenteral nutrition. Treatment is usually by the oral administration of up to 100 mmol of phosphate daily.
Approximately two-thirds of ingested phosphate is absorbed from the gastrointestinal tract, virtually all of it being excreted in the urine, and the remainder is excreted in the feces. Excessive administration of phosphate, particularly intravenously, rectally, or in patients with renal failure, can cause hyperphosphatemia that may lead to hypocalcemia or other severe electrolyte imbalances. Adverse effects occur less frequently following oral consumption, although phosphates act as mild saline laxatives when administered orally or rectally (2–4 g of monobasic sodium phosphate in an aqueous solution is used as a laxative). Consequently, gastrointestinal disturbances including diarrhea, nausea, and vomiting may occur following the use of monobasic sodium phosphate as an excipient in oral formulations. However, the level of monobasic sodium phosphate used as an excipient in a pharmaceutical formulation is not usually associated with adverse effects.
LD50 (rat, IM): 0.25 g/kg(10)
LD50 (rat, oral): 8.29 g/kg
хранилище
Monobasic sodium phosphate is chemically stable, although it is slightly deliquescent. On heating at 100°C, the dihydrate loses all of its water of crystallization. On further heating, it melts with decomposition at 205℃, forming sodium hydrogen pyrophosphate, Na2H2P2O7. At 250℃ it leaves a final residue of sodium metaphosphate, NaPO3.Aqueous solutions are stable and may be sterilized by autoclaving.
Monobasic sodium phosphate should be stored in an airtight container in a cool, dry place.
Несовместимости
Monobasic sodium phosphate is an acid salt and is therefore generally incompatible with alkaline materials and carbonates; aqueous solutions of monobasic sodium phosphate are acidic and will cause carbonates to effervesce.Monobasic sodium phosphate should not be administered concomitantly with aluminum, calcium, or magnesium salts since they bind phosphate and could impair its absorption from the gastrointestinal tract. Interaction between calcium and phosphate, leading to the formation of insoluble calcium phosphate precipitates, is possible in parenteral admixtures.
Регуляторный статус
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (injections; infusions; ophthalmic, oral, topical, and vaginal preparations). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.Sodium phosphate monobasic monohydrate запасные части и сырье
Sodium phosphate monobasic monohydrate поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +8617732866630 | China | 18147 | 58 | |
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 | |
| +86-371-66670886 | China | 19902 | 58 | |
| +8618805133257 | China | 132 | 60 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 008657128800458; +8615858145714 |
China | 7738 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 |
Sodium phosphate monobasic monohydrate Обзор)
- Sodium dihydrogen orthophosphate
- Дигидрофосфат натрия двуводный (USP
- Potassium dihydrogen orthophosphate
- Аммония дигидрофосфат
- Бензил изоцианид
- Метил isocyanoacetate
- Натрий пирофосфат декагидрат
- Этил изоцианоацета
- Фенилселенол
- Adenosine 2′:3′-cyclic monophosphate sodium salt
1of4