Ketones spices
Organic compounds with both ends of carbonyl connected with the hydrocarbon are called ketone. When the hydrocarbon group connected to both ends of the carbonyl group is aliphatic hydrocarbyl group, the compound belongs to aliphatic ketone; when there is at least one end of the carbonyl group connected with aromatic hydrocarbon group, the compound is called aromatic ketone. According to the number of carbonyl groups contained in the molecule, ketones can be divided into monoketone and diketones. Saturated monoaldehyde and ketone having the same number of carbon atoms are isomers with each other.
There are nearly 100 kinds of ketones spices with the chemical properties being generally stable but with varying degree of color change. Among them, aliphatic ketone has strong aroma with some individual species being used in only a small amount in the formulation of daily flavor. Aromatic ketones are only used in small quantities in individual flavors. Among those cyclic ketones consisting of 5 or 6 carbons or multiple carbons, widely used varieties include ionone, methyl ionone and damascone. Macrocyclic ketone containing more than 15 carbons is a composition of the natural animal spices. However, owning to the high price of synthetic products, it is only used at high-grade perfume fragrance.
Lower aliphatic ketones (both hydrocarbon groups are aliphatic hydrocarbons) are liquid of special odor smell. High-grade ketones are more fragrant, being easily soluble in water, ether and ethanol. With the increase in carbon number, they have decreased solubility but increased viscosity. Ketones of more than six carbon atoms are generally insoluble in water; higher aliphatic ketones are solids; aromatic ketones (both hydrocarbon groups are aromatic) are mostly solids. The simplest mono-ketone is acetone; the simplest mono-aromatic ketone is benzophenone. Lower ketone has a lower boiling point than alcohol of similar relative molecular mass, which is mainly due to hydrogen bonds can’t form between ketone molecules, thus having no association effect. But the boiling point of the higher ketone is gradually close to that of the alcohol of similar relative molecular mass.
Ketone has relatively active chemical activity, this is because the carbon-oxygen double bond of carbonyl group contains one α bond and one π bond, being an unsaturated polar bond. The oxygen atom has a stronger negative charge than the carbon, thus strongly attracting electron, enhancing the density of the electronic cloud around the oxygen atom while carbon atom has lower electronic cloud density, thus leading to a certain polarity with carbon atoms become positive carbon and oxygen atoms become negative oxygen ions. Compare those two, the former one is more active than the latter one, and is susceptible to the attack of nucleophilic reagents carrying charges or lone electron pair, inducing the occurrence of addition reaction, so the addition of ketones is mostly nucleophilic addition. For example, ketone and nucleophiles such as HCN, NH2OH and NH2NH2 can easily have additions reaction and these addition reactions are mostly reversible.
- Structure:
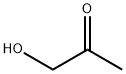
- Chemical Name:Hydroxyacetone
- CAS:116-09-6
- MF:C3H6O2
- Structure:
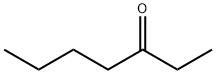
- Chemical Name:3-Heptanone
- CAS:106-35-4
- MF:C7H14O
- Structure:
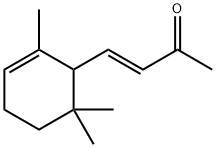
- Chemical Name:alpha-Ionone
- CAS:127-41-3
- MF:C13H20O
- Structure:
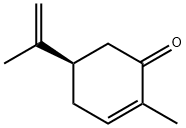
- Chemical Name:L(-)-Carvone
- CAS:6485-40-1
- MF:C10H14O
- Structure:
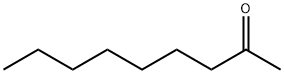
- Chemical Name:2-Nonanone
- CAS:821-55-6
- MF:C9H18O
- Structure:
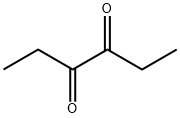
- Chemical Name:3,4-Hexanedione
- CAS:4437-51-8
- MF:C6H10O2
- Structure:
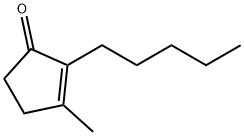
- Chemical Name:2-Pentyl-3-methyl-2-cyclopenten-1-one
- CAS:1128-08-1
- MF:C11H18O
- Structure:
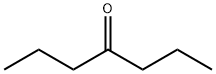
- Chemical Name:4-Heptanone
- CAS:123-19-3
- MF:C7H14O
- Structure:
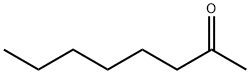
- Chemical Name:2-Octanone
- CAS:111-13-7
- MF:C8H16O
- Structure:
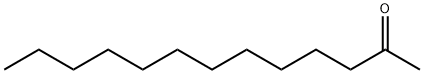
- Chemical Name:2-Tridecanone
- CAS:593-08-8
- MF:C13H26O
- Structure:
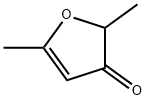
- Chemical Name:2,5-Dimethyl-3(2H)-furanone
- CAS:14400-67-0
- MF:C6H8O2
- Structure:
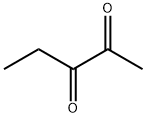
- Chemical Name:2,3-Pentanedione
- CAS:600-14-6
- MF:C5H8O2
- Structure:
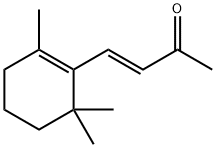
- Chemical Name:β-Lonone
- CAS:79-77-6
- MF:C13H20O
- Structure:
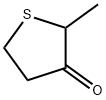
- Chemical Name:Dihydro-2-methyl-3(2H)-thiophenone
- CAS:13679-85-1
- MF:C5H8OS
- Structure:
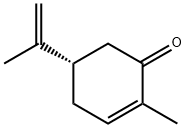
- Chemical Name:D(+)-Carvone
- CAS:2244-16-8
- MF:C10H14O
- Structure:
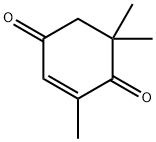
- Chemical Name:2,6,6-Trimethyl-2-cyclohexene-1,4-dione
- CAS:1125-21-9
- MF:C9H12O2
- Structure:
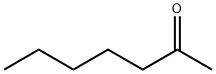
- Chemical Name:2-Heptanone
- CAS:110-43-0
- MF:C7H14O
- Structure:
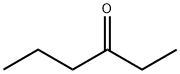
- Chemical Name:3-Hexanone
- CAS:589-38-8
- MF:C6H12O
- Structure:
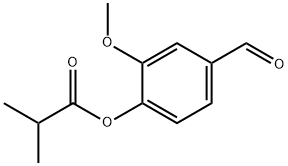
- Chemical Name:Vanillin isobutyrate
- CAS:20665-85-4
- MF:C12H14O4
- Structure:
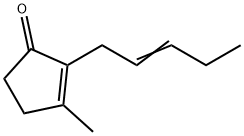
- Chemical Name:Jasmone
- CAS:488-10-8
- MF:C11H16O
- Structure:
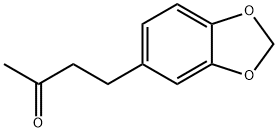
- Chemical Name:Piperonyl acetone
- CAS:55418-52-5
- MF:C11H12O3
- Structure:
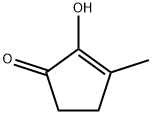
- Chemical Name:Methyl cyclopentenolone
- CAS:80-71-7
- MF:C6H8O2
- Structure:
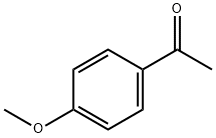
- Chemical Name:4'-Methoxyacetophenone
- CAS:100-06-1
- MF:C9H10O2
- Structure:
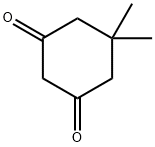
- Chemical Name:Dimedone
- CAS:126-81-8
- MF:C8H12O2
- Structure:
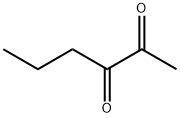
- Chemical Name:2,3-HEXANEDIONE
- CAS:3848-24-6
- MF:C6H10O2
- Structure:
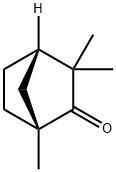
- Chemical Name:(-)-FENCHONE
- CAS:7787-20-4
- MF:C10H16O
- Structure:
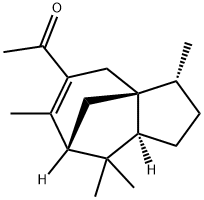
- Chemical Name:Methyl Cedryl Ketone
- CAS:32388-55-9
- MF:C17H26O
- Structure:
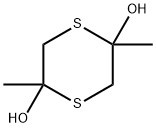
- Chemical Name:Dimeric mercapto propanone
- CAS:55704-78-4
- MF:C6H12O2S2
- Structure:
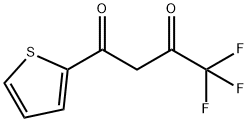
- Chemical Name:Thenoyltrifluoroacetone
- CAS:326-91-0
- MF:C8H5F3O2S
- Structure:
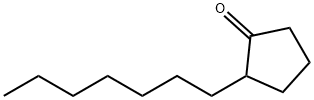
- Chemical Name:2-N-HEPTYLCYCLOPENTANONE
- CAS:137-03-1
- MF:C12H22O
- Structure:
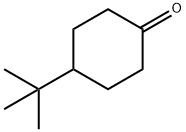
- Chemical Name:4-tert-Butylcyclohexanone
- CAS:98-53-3
- MF:C10H18O
- Structure:
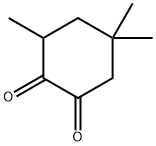
- Chemical Name:3,5,5-Trimethylcyclohexane-1,2-dione
- CAS:57696-89-6
- MF:C9H14O2
- Structure:
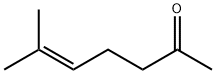
- Chemical Name:6-Methyl-5-hepten-2-one
- CAS:110-93-0
- MF:C8H14O
- Structure:
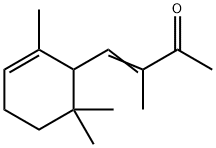
- Chemical Name:ALPHA-ISO-METHYLIONONE
- CAS:127-51-5
- MF:C14H22O
- Structure:
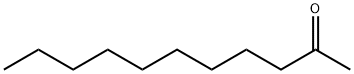
- Chemical Name:2-Undecanone
- CAS:112-12-9
- MF:C11H22O
- Structure:
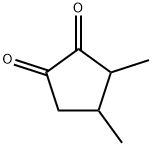
- Chemical Name:3,4-dimethyl 2-hydroxy-2-cyclopenten-1-one
- CAS:13494-06-9
- MF:C7H10O2
- Structure:
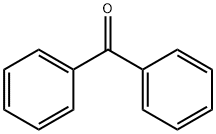
- Chemical Name:Benzophenone
- CAS:119-61-9
- MF:C13H10O
- Structure:
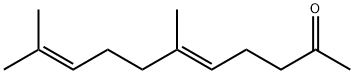
- Chemical Name:Geranylacetone
- CAS:3796-70-1
- MF:C13H22O
- Structure:
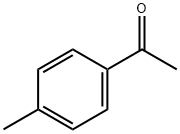
- Chemical Name:4'-Methylacetophenone
- CAS:122-00-9
- MF:C9H10O
- Structure:
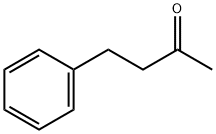
- Chemical Name:Benzylacetone
- CAS:2550-26-7
- MF:C10H12O
- Structure:
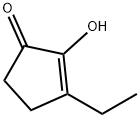
- Chemical Name:3-Ethyl-2-hydroxy-2-cyclopenten-1-one
- CAS:21835-01-8
- MF:C7H10O2
- Structure:
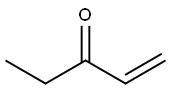
- Chemical Name:Ethyl vinyl ketone
- CAS:1629-58-9
- MF:C5H8O
- Structure:
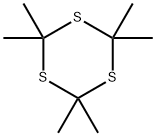
- Chemical Name:2,2,4,4,6,6-Hexamethyl-S-trithiane
- CAS:828-26-2
- MF:C9H18S3
- Structure:
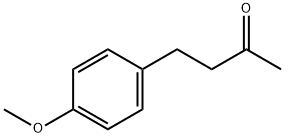
- Chemical Name:4-(4-Methoxyphenyl)-2-butanone
- CAS:104-20-1
- MF:C11H14O2
- Structure:
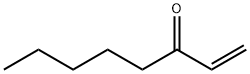
- Chemical Name:1-Octen-3-one
- CAS:4312-99-6
- MF:C8H14O
- Structure:
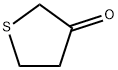
- Chemical Name:Tetrahydrothiophen-3-one
- CAS:1003-04-9
- MF:C4H6OS
- Structure:
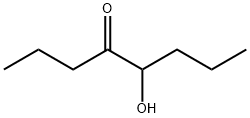
- Chemical Name:5-Hydroxy-4-octanone
- CAS:496-77-5
- MF:C8H16O2
- Structure:
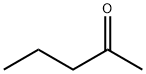
- Chemical Name:2-Pentanone
- CAS:107-87-9
- MF:C5H10O
- Structure:
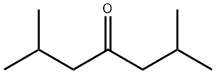
- Chemical Name:2,6-Dimethyl-4-heptanone
- CAS:108-83-8
- MF:C9H18O
- Structure:
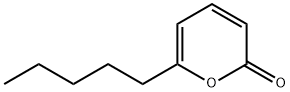
- Chemical Name:6-Pentyl-2H-pyran-2-one
- CAS:27593-23-3
- MF:C10H14O2
- Structure:
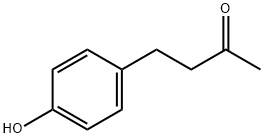
- Chemical Name:Raspberry Ketone
- CAS:5471-51-2
- MF:C10H12O2
- Structure:
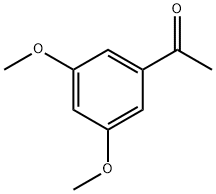
- Chemical Name:3',5'-Dimethoxyacetophenone
- CAS:39151-19-4
- MF:C10H12O3
- Structure:
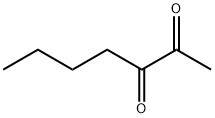
- Chemical Name:2,3-HEPTANEDIONE
- CAS:96-04-8
- MF:C7H12O2
- Structure:
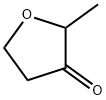
- Chemical Name:2-Methyltetrahydrofuran-3-one
- CAS:3188-00-9
- MF:C5H8O2
- Structure:
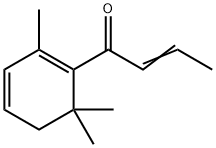
- Chemical Name:Damascenone
- CAS:23696-85-7
- MF:C13H18O
- Structure:
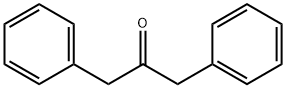
- Chemical Name:1,3-Diphenylacetone
- CAS:102-04-5
- MF:C15H14O
- Structure:
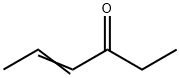
- Chemical Name:4-HEXEN-3-ONE
- CAS:2497-21-4
- MF:C6H10O
- Structure:
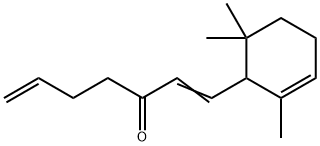
- Chemical Name:Allyl-α-ionone
- CAS:79-78-7
- MF:C16H24O
- Structure:
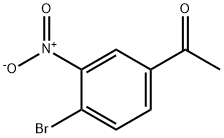
- Chemical Name:4'-Bromo-3'-nitroacetophenone
- CAS:18640-58-9
- MF:C8H6BrNO3
- Structure:
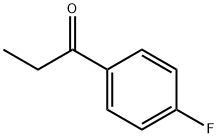
- Chemical Name:4'-Fluoropropiophenone
- CAS:456-03-1
- MF:C9H9FO
- Structure:
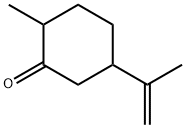
- Chemical Name:(+)-DIHYDROCARVONE
- CAS:7764-50-3
- MF:C10H16O
- Structure:
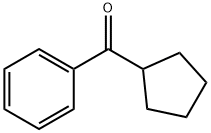
- Chemical Name:CYCLOPENTYL PHENYL KETONE
- CAS:5422-88-8
- MF:C12H14O
- Structure:
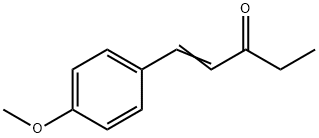
- Chemical Name:1-(4-Methoxyphenyl)-1-penten-3-one
- CAS:104-27-8
- MF:C12H14O2
- Structure:
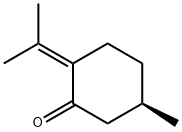
- Chemical Name:(+)-PULEGONE
- CAS:89-82-7
- MF:C10H16O
- Structure:
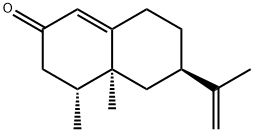
- Chemical Name:NOOTKATONE
- CAS:4674-50-4
- MF:C15H22O
- Structure:
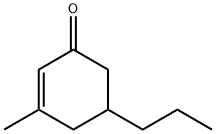
- Chemical Name:Celery ketone
- CAS:3720-16-9
- MF:C10H16O
- Structure:
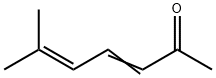
- Chemical Name:6-METHYL-3,5-HEPTADIEN-2-ONE
- CAS:1604-28-0
- MF:C8H12O
- Structure:
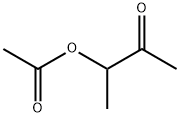
- Chemical Name:3-ACETOXY-2-BUTANONE
- CAS:4906-24-5
- MF:C6H10O3
- Structure:
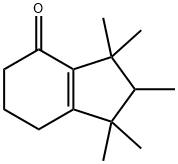
- Chemical Name:CASHMERAN
- CAS:33704-61-9
- MF:C14H22O
- Structure:
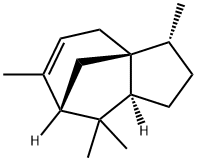
- Chemical Name:(-)-ALPHA-CEDRENE
- CAS:469-61-4
- MF:C15H24
- Structure:
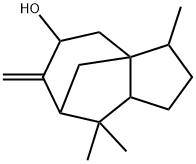
- Chemical Name:Cedrenol
- CAS:28231-03-0
- MF:C15H24O
- Structure:
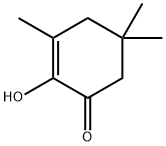
- Chemical Name:3,5,5-TRIMETHYLCYCLOHEXANE-1,2-DIONE
- CAS:4883-60-7
- MF:C9H14O2
- Structure:
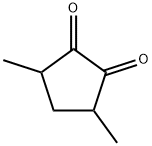
- Chemical Name:3,5-Dimethyl-1,2-cyclopentanedione
- CAS:13494-07-0
- MF:C7H10O2
- Structure:
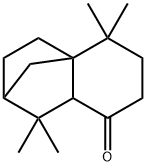
- Chemical Name:Isolongifolone
- CAS:23787-90-8
- MF:C15H24O
- Structure:
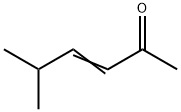
- Chemical Name:5-METHYL-3-HEXEN-2-ONE
- CAS:5166-53-0
- MF:C7H12O
- Structure:
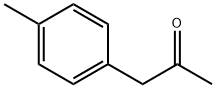
- Chemical Name:4-METHYLPHENYLACETONE
- CAS:2096-86-8
- MF:C10H12O
- Structure:
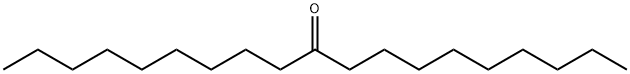
- Chemical Name:10-NONADECANONE
- CAS:504-57-4
- MF:C19H38O
- Structure:
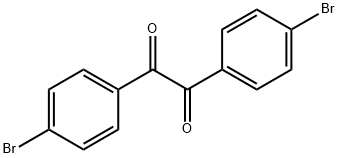
- Chemical Name:4,4'-DIBROMOBENZIL
- CAS:35578-47-3
- MF:C14H8Br2O2
- Structure:
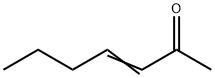
- Chemical Name:3-HEPTEN-2-ONE
- CAS:1119-44-4
- MF:C7H12O
- Structure:
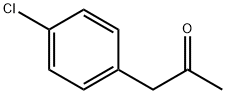
- Chemical Name:4-Chlorophenylacetone
- CAS:5586-88-9
- MF:C9H9ClO
- Structure:
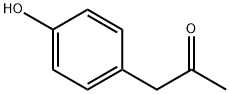
- Chemical Name:4-Hydroxyphenylacetone
- CAS:770-39-8
- MF:C9H10O2
- Structure:
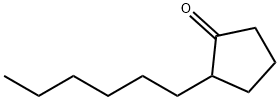
- Chemical Name:2-N-HEXYLCYCLOPENTANONE
- CAS:13074-65-2
- MF:C11H20O
- Structure:
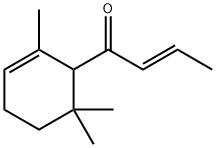
- Chemical Name:(E)-1-(2,6,6-TRIMETHYL-CYCLOHEX-2-ENYL)-BUT-2-EN-1-ONE
- CAS:24720-09-0
- MF:C13H20O
- Structure:
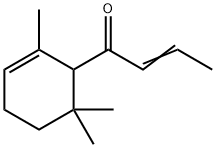
- Chemical Name:Alpha-Damascone
- CAS:43052-87-5
- MF:C13H20O
- Structure:
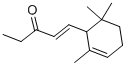
- Chemical Name:METHYLIONONE
- CAS:1335-46-2
- MF:C14H22O
- Chemical Name:CEDRENE, (-)-alpha-(SG)
- CAS:
- MF:
- Structure:
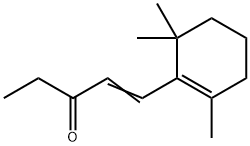
- Chemical Name:BETA-N-METHYLIONONE
- CAS:127-43-5
- MF:C14H22O
- Structure:
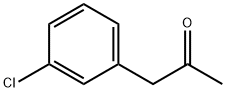
- Chemical Name:3-CHLOROPHENYLACETONE
- CAS:14123-60-5
- MF:C9H9ClO
- Structure:
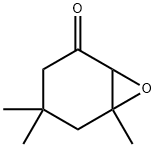
- Chemical Name:Isophorone oxide
- CAS:10276-21-8
- MF:C9H14O2
- Structure:
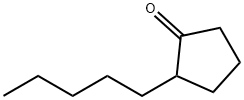
- Chemical Name:2-N-PENTYLCYCLOPENTANONE
- CAS:4819-67-4
- MF:C10H18O
- Structure:
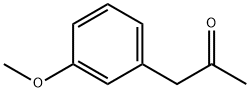
- Chemical Name:3-METHOXYPHENYLACETONE
- CAS:3027-13-2
- MF:C10H12O2
- Structure:
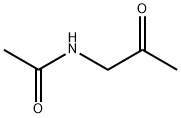
- Chemical Name:1-ACETAMIDO-ACETONE
- CAS:7737-16-8
- MF:C5H9NO2
- Structure:
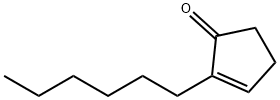
- Chemical Name:ISOJASMONE
- CAS:95-41-0
- MF:C11H18O
- Structure:
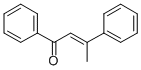
- Chemical Name:1,3-DIPHENYL-2-BUTEN-1-ONE
- CAS:1322-90-3
- MF:C16H14O
- Structure:
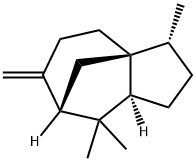
- Chemical Name:(+)-BETA-CEDRENE
- CAS:546-28-1
- MF:C15H24
- Structure:
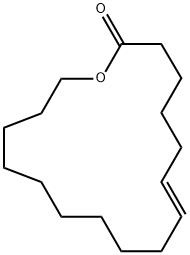
- Chemical Name:AMBRETTOLIDE
- CAS:7779-50-2
- MF:C16H28O2
- Structure:
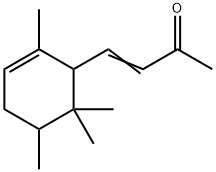
- Chemical Name:IRONE
- CAS:79-69-6
- MF:C14H22O
- Structure:
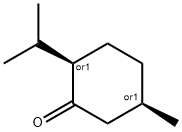
- Chemical Name:ISOMENTHONE
- CAS:491-07-6
- MF:C10H18O
- Structure:
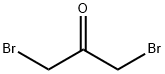
- Chemical Name:1,3-DIBROMOACETONE
- CAS:816-39-7
- MF:C3H4Br2O
- Structure:
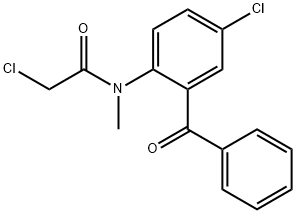
- Chemical Name:N-(2-benzoyl-4-chlorophenyl)-2-chloro-N-methylacetamide
- CAS:6021-21-2
- MF:C16H13Cl2NO2