CLEAR COLOURLESS TO SLIGHTLY YELLOW LIQUID
Reported to be found in many essential oils, including those of Thuja plicata, T.occiden-talis, T.standi shii, Russian anise, fennel, a few Artemisia varieties (A. frigida, A . verlotorum and A . santolinaefolia), Lavandula stoechas and L. burmannii. The highest levels (12-19%) are found in fennel oil (Fenarolis Handbook of Flavor Ingredients, 1975; Gildemeister & Hoffman, 1963).
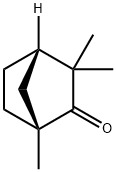
(1R,4S)-1,3,3-Trimethylbicyclo[2.2.1]heptan-2-one also known more commonly as (-)-Fenchone is a chiral intermediate of Fenchone and is currently being used for studies ranging from inhibitory effects of monoterpenes on human TRPA1 and odorant receptor of the malaria vector Anopheles gambiae.
ChEBI: A fenchone that has (1R,4S)-stereochemistry. It is a constituent of the essential oils obtained from fennel.
By isolation from cedar leaf oil (Thuja oil) or by various synthetic methods (Arctander, 1969).
(1R)-(-)-Fenchone is a bridged bicyclic ketone found in fennel oil and thuja oil.
Flammability and Explosibility
Not classified
In mice, fenchone injected sc in sesame oil produced clonic convulsions at non-lethal
doses, with a median convulsive dose (CD50) of 1133 mg/kg, and a dose of 500 mg/kg given sc
was an effective arousal agent, reducing the hexobarbitone sleep time (Wenzel & Ross, 1957). In rats, an ip dose of 500 mg/kg had no effect on pentobarbitone-depressed respiration, while ip doses
of 50-400 mg/kg increased running activity but did not affect total activity (Wenzel & Ross, 1957).
Fenchone showed some antispasmodic action on excised mouse intestine (Haginiwa, Harada &
Morishita, 1963), and at 260 mmol/kg showed good choleretic properties of moderately long duration
when given orally in olive oil to rats (Mrsdorf, 1966). Thomas (1958) reported that it acted as
a central nervous system stimulant. Fenchone has been used medically as a counter-irritant (Merck
Index, 1968).
Rimini (1901 & 1909) showed that, in the dog, fenchone was probably oxidized to 4-hydroxyfenchone. Reinartz & Zanke (1936) showed that there were other products. They separated, as lead salts, the glucuronides from the urine of dogs receiving d-fenchone. The lead was removed with sulphuric acid and the resulting solution was hydrolysed. In the resulting mixture of hydroxyfen chones, the presence of 4- and 5-hydroxyfenchones and 7r-apofenchone-3-carboxylic acid was demon strated (Williams, 1959).
Purification is as for the (+)-enantiomer above and should have the same physical properties except for opposite optical rotations. UV has max 285nm ( 12.29). [Braun & Jacob Chem Ber 66 1461 1933, UV: Ohloff et al. Chem Ber 90 106 1957.] [Beilstein 7 III 392, 7 IV 212.]