Aromatic hydrocarbons
Aromatic hydrocarbon refers to the closed-chain hydrocarbon containing a benzene ring or having the basic characteristics of the aromatic compound. It can be divided into mono-cyclic aromatic hydrocarbon, polycyclic aromatic hydrocarbon and non-benzene aromatic hydrocarbons. For monocyclic aromatic hydrocarbon, its molecule contains only one benzene ring, such as toluene, xylene, ethylbenzene, styrene, benzene and acetylene.
Polycyclic aromatic hydrocarbon contains two or more benzene rings. According to the interconnected ways of benzene ring, it can be divided into:
1 polybenzo-aliphatic hydrocarbons including diphenylmethane, triphenylmethane, stilbene, styrene and so on;
2 Biphenyl and polybenzene, such as biphenyl, terphenyl and tetraphenyl, etc;
3 polycyclic aromatic hydrocarbons such as naphthalene, anthracene, phenanthrene, indene, fluorene, acenaphthene, pyrene, coronene and so on;
Non-aromatic hydrocarbon is the hydrocarbon containing certain aromatic property but containing no benzene ring such as azulene and annulene. It is easy to have substitution reaction while being not easy to have addition reaction with the ring being not easy to be broken.
The natural existing aromatic hydrocarbon is mainly is mainly obtained through from the distillate of coal tar and the aromatization product of oil. The main component of coal tar is aromatic hydrocarbon; the main component of various kinds of crude oil of petroleum is aliphatic hydrocarbons with aromatic hydrocarbons only accounting for only 0.2% to 7.4%, but the acute and chronic toxicity of oil is mainly aromatic hydrocarbons, in particular caused by the soluble aromatic hydrocarbons such as naphthalene, phenanthrene contained in it. It can be used as solvent and chemical raw materials.
Benzene is the basic structure of aromatic hydrocarbon, usually using the structure formula of six-membered ring containing three conjugated double bonds, which was proposed at 1865 by the German chemist F.A. Kekule, being called as Kekule formula. From the composition point of view, benzene is an unsaturated hydrocarbon, but it is not easy to be subject to oxidation and addition reaction while being easy to participate in substitution reaction, and there is also special toughness (see "aromatic"). According to the hybrid orbital theory, each carbon atoms contained in the benzene molecule is sp2 hybridized with each of them having two sp2 hybrid orbitals overlapped with the sp2 hybrid orbitals from two adjacent carbon atoms, forming a C-Cσ bond (see Fig. 1), and with a sp2 hybrid orbital overlapping with the 1s orbital of the hydrogen atom, forming a C-Hσ bond.
The simplest aromatic hydrocarbon is benzene. People originally discovered benzene and other aromatic compounds from coal tar, and thereby, coal tar, for quite a long time, had been the major source of aromatics. But the yield from coal tar is only 3% of that of the coal; moreover, the content of various kinds of aromatic compound contained in coal tar is only about 0.3% of that of the coal. Therefore, people have mainly manufactured aromatics from petroleum processing system since the 1940s. Aromatics are all liquid or solid. It is insoluble in water but soluble in organic solvents. It has aromaticity and is not easy to participate in the addition reaction, but is easily subject to substitution reaction, and is relatively stable to heat and oxidant. Aromatic hydrocarbons (especially single-ring aromatic hydrocarbon) are important chemical raw materials (for example, xylene is the raw material for the manufacturing of polyester), many kinds of liquid aromatic hydrocarbon can be used as solvents (for example, paint is easily soluble in aromatic hydrocarbons). However, when the liquid aromatic hydrocarbon has been in contact with the skin for several times, due to dehydration and degreasing, can cause dermatitis. The steam of the aromatic hydrocarbons has a stronger stimulation effect on the mucosa than the aliphatic hydrocarbon. In particular, benzene vapor has a special destroying effect on the hematopoietic function. However, animal tests have shown that alkylbenzene has no such damaging effects.
- Structure:
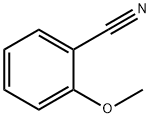
- Chemical Name:2-Methoxybenzonitrile
- CAS:6609-56-9
- MF:C8H7NO
- Structure:
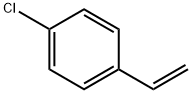
- Chemical Name:4-Chlorostyrene
- CAS:1073-67-2
- MF:C8H7Cl
- Structure:
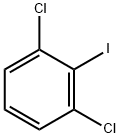
- Chemical Name:2,6-DICHLOROIODOBENZENE
- CAS:19230-28-5
- MF:C6H3Cl2I
- Structure:
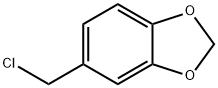
- Chemical Name:Piperonyl chloride
- CAS:20850-43-5
- MF:C8H7ClO2
- Structure:
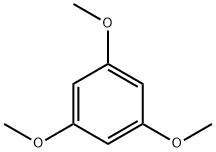
- Chemical Name:1,3,5-Trimethoxybenzene
- CAS:621-23-8
- MF:C9H12O3
- Structure:
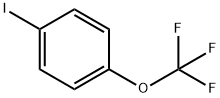
- Chemical Name:1-Iado-4-(trifluoromethoxy)benzene
- CAS:103962-05-6
- MF:C7H4F3IO
- Structure:
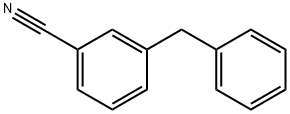
- Chemical Name:2-Cyano-4'-methylbiphenyl
- CAS:93717-55-6
- MF:C14H11N
- Structure:
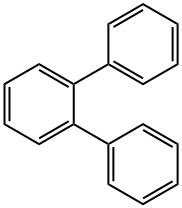
- Chemical Name:o-Terphenyl
- CAS:84-15-1
- MF:C18H14
- Structure:
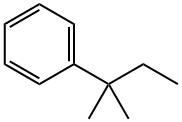
- Chemical Name:tert-Amylbenzene
- CAS:2049-95-8
- MF:C11H16
- Structure:
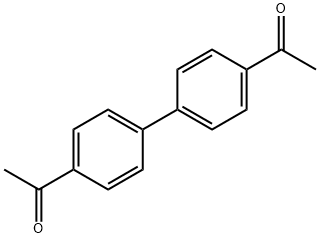
- Chemical Name:4,4'-Diacetylbiphenyl
- CAS:787-69-9
- MF:C16H14O2
- Structure:
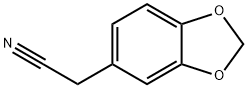
- Chemical Name:3,4-(Methylenedioxy)phenylacetonitrile
- CAS:4439-02-5
- MF:C9H7NO2
- Structure:
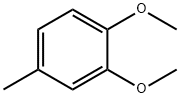
- Chemical Name:3,4-Dimethoxytoluene
- CAS:494-99-5
- MF:C9H12O2
- Structure:
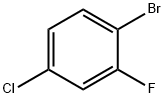
- Chemical Name:1-Bromo-4-chloro-2-fluorobenzene
- CAS:1996-29-8
- MF:C6H3BrClF
- Structure:
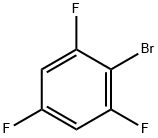
- Chemical Name:1-Bromo-2,4,6-trifluorobenzene
- CAS:2367-76-2
- MF:C6H2BrF3
- Structure:
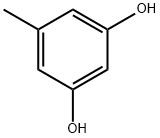
- Chemical Name:Orcinol
- CAS:504-15-4
- MF:C7H8O2
- Structure:
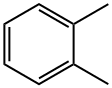
- Chemical Name:o-Xylene
- CAS:95-47-6
- MF:C8H10
- Structure:
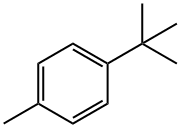
- Chemical Name:4-tert-Butyltoluene
- CAS:98-51-1
- MF:C11H16
- Structure:
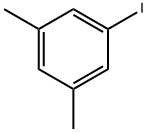
- Chemical Name:1-Iodo-3,5-dimethylbenzene
- CAS:22445-41-6
- MF:C8H9I
- Structure:
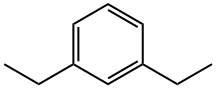
- Chemical Name:1,3-Diethylbenzene
- CAS:141-93-5
- MF:C10H14
- Structure:
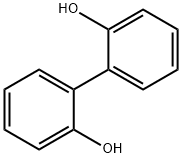
- Chemical Name:2,2'-Biphenol
- CAS:1806-29-7
- MF:C12H10O2
- Structure:
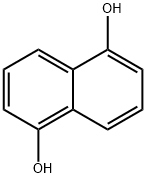
- Chemical Name:1,5-Dihydroxy naphthalene
- CAS:83-56-7
- MF:C10H8O2
- Structure:
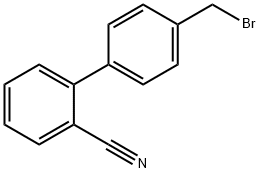
- Chemical Name:4-Bromomethyl-2-cyanobiphenyl
- CAS:114772-54-2
- MF:C14H10BrN
- Structure:
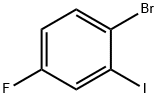
- Chemical Name:1-Bromo-4-fluoro-2-iodobenzene
- CAS:202865-72-3
- MF:C6H3BrFI
- Structure:
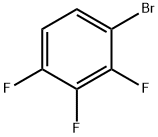
- Chemical Name:2,3,4-Trifluorobromobenzene
- CAS:176317-02-5
- MF:C6H2BrF3
- Structure:
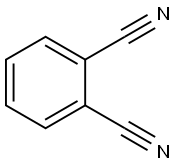
- Chemical Name:Phthalonitrile
- CAS:91-15-6
- MF:C8H4N2
- Structure:
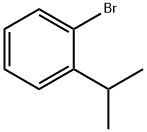
- Chemical Name:1-Bromo-2-(1-methylethyl)benzene
- CAS:7073-94-1
- MF:C9H11Br
- Structure:
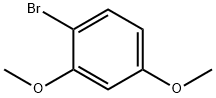
- Chemical Name:1-Bromo-2,4-dimethoxybenzene
- CAS:17715-69-4
- MF:C8H9BrO2
- Structure:
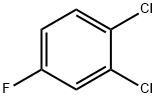
- Chemical Name:1,2-Dichloro-4-fluorobenzene
- CAS:1435-49-0
- MF:C6H3Cl2F
- Structure:
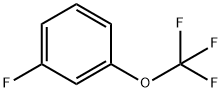
- Chemical Name:3-(Trifluoromethoxy)fluorobenzene
- CAS:1077-01-6
- MF:C7H4F4O
- Structure:
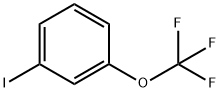
- Chemical Name:3-(Trifluoromethoxy)iodobenzene
- CAS:198206-33-6
- MF:C7H4F3IO
- Structure:
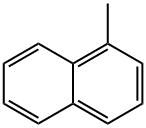
- Chemical Name:1-Methylnaphthalene
- CAS:90-12-0
- MF:C11H10
- Structure:
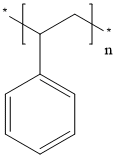
- Chemical Name:Polystyrene
- CAS:9003-53-6
- MF:[CH2CH(C6H5)]n
- Structure:
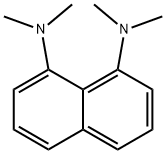
- Chemical Name:1,8-Bis(dimethylamino)naphthalene
- CAS:20734-58-1
- MF:C14H18N2
- Structure:
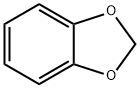
- Chemical Name:1,3-Benzodioxole
- CAS:274-09-9
- MF:C7H6O2
- Structure:
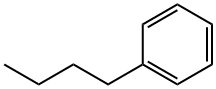
- Chemical Name:Butylbenzene
- CAS:104-51-8
- MF:C10H14
- Structure:
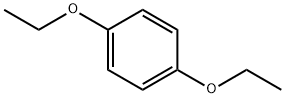
- Chemical Name:1,4-Diethoxybenzene
- CAS:122-95-2
- MF:C10H14O2
- Structure:
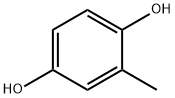
- Chemical Name:2-Methylhydroquinone
- CAS:95-71-6
- MF:C7H8O2
- Structure:
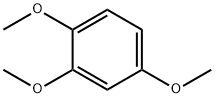
- Chemical Name:1,2,4-Trimethoxybenzene
- CAS:135-77-3
- MF:C9H12O3
- Structure:
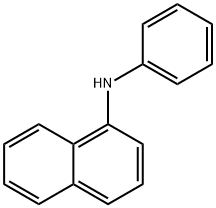
- Chemical Name:N-Phenyl-1-naphthylamine
- CAS:90-30-2
- MF:C16H13N
- Structure:
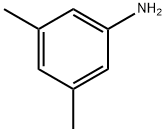
- Chemical Name:3,5-Dimethylaniline
- CAS:108-69-0
- MF:C8H11N
- Structure:
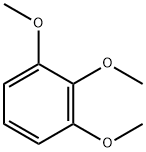
- Chemical Name:1,2,3-Trimethoxybenzene
- CAS:634-36-6
- MF:C9H12O3
- Structure:
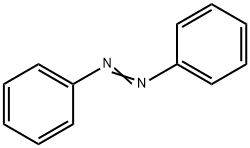
- Chemical Name:Azobenzene
- CAS:103-33-3
- MF:C12H10N2
- Structure:
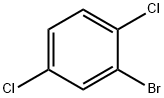
- Chemical Name:2-Bromo-1,4-dichlorobenzene
- CAS:1435-50-3
- MF:C6H3BrCl2
- Structure:
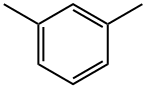
- Chemical Name:m-Xylene
- CAS:108-38-3
- MF:C8H10
- Structure:
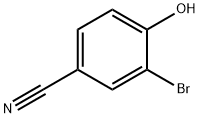
- Chemical Name:3-BROMO-4-HYDROXYBENZONITRILE
- CAS:2315-86-8
- MF:C7H4BrNO
- Structure:
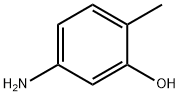
- Chemical Name:5-Amino-o-cresol
- CAS:2835-95-2
- MF:C7H9NO
- Structure:
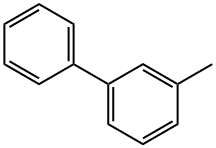
- Chemical Name:3-Phenyltoluene
- CAS:643-93-6
- MF:C13H12
- Structure:
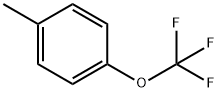
- Chemical Name:4-Trifluoromethoxytoluene
- CAS:706-27-4
- MF:C8H7F3O
- Structure:
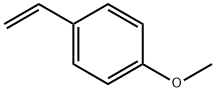
- Chemical Name:4-Methoxystyrene
- CAS:637-69-4
- MF:C9H10O
- Structure:
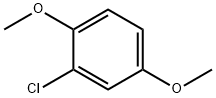
- Chemical Name:2-Chloro-1,4-dimethoxybenzene
- CAS:2100-42-7
- MF:C8H9ClO2
- Structure:
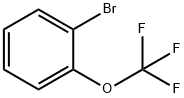
- Chemical Name:2-(Trifluoromethoxy)bromobenzene
- CAS:64115-88-4
- MF:C7H4BrF3O
- Structure:
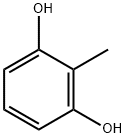
- Chemical Name:2-Methylresorcinol
- CAS:608-25-3
- MF:C7H8O2
- Structure:
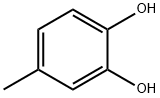
- Chemical Name:4-Methylcatechol
- CAS:452-86-8
- MF:C7H8O2
- Structure:
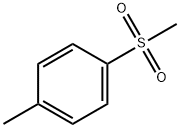
- Chemical Name:1-Methyl-4-(methylsulfonyl)-benzene
- CAS:3185-99-7
- MF:C8H10O2S
- Structure:
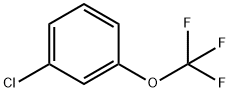
- Chemical Name:3-(Trifluoromethoxy)chlorobenzene
- CAS:772-49-6
- MF:C7H4ClF3O
- Structure:
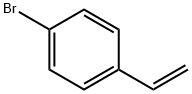
- Chemical Name:4-Bromostyrene
- CAS:2039-82-9
- MF:C8H7Br
- Structure:
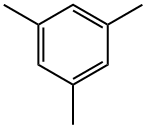
- Chemical Name:Mesitylene
- CAS:108-67-8
- MF:C9H12
- Structure:
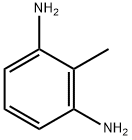
- Chemical Name:2,6-Diaminotoluene
- CAS:823-40-5
- MF:C7H10N2
- Structure:
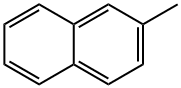
- Chemical Name:2-Methylnaphthalene
- CAS:91-57-6
- MF:C11H10
- Structure:
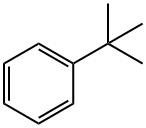
- Chemical Name:tert-Butylbenzene
- CAS:98-06-6
- MF:C10H14
- Structure:
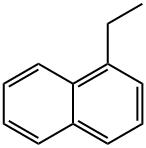
- Chemical Name:1-ETHYLNAPHTHALENE
- CAS:1127-76-0
- MF:C12H12
- Structure:
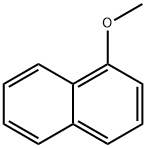
- Chemical Name:1-Methoxynaphthalene
- CAS:2216-69-5
- MF:C11H10O
- Structure:
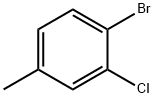
- Chemical Name:4-BROMO-3-CHLOROTOLUENE
- CAS:6627-51-6
- MF:C7H6BrCl
- Structure:
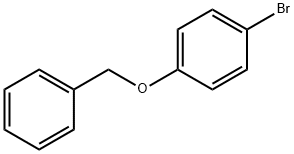
- Chemical Name:4-Benzyloxybromobenzene
- CAS:6793-92-6
- MF:C13H11BrO
- Structure:
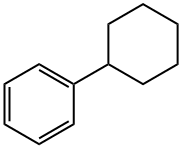
- Chemical Name:Cyclohexylbenzene
- CAS:827-52-1
- MF:C12H16
- Structure:
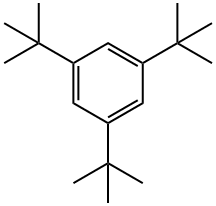
- Chemical Name:1,3,5-Tri-tert-butylbenzene
- CAS:1460-02-2
- MF:C18H30
- Structure:
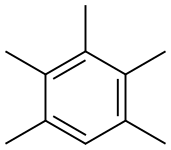
- Chemical Name:Pentamethylbenzene
- CAS:700-12-9
- MF:C11H16
- Structure:
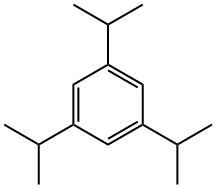
- Chemical Name:1,3,5-Triisopropylbenzene
- CAS:717-74-8
- MF:C15H24
- Structure:
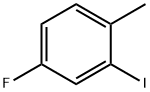
- Chemical Name:4-FLUORO-2-IODOTOLUENE
- CAS:13194-67-7
- MF:C7H6FI
- Structure:
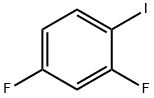
- Chemical Name:2,4-Difluoroiodobenzene
- CAS:2265-93-2
- MF:C6H3F2I
- Structure:
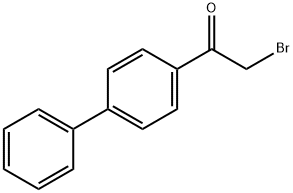
- Chemical Name:2-BROMO-4'-PHENYLACETOPHENONE
- CAS:135-73-9
- MF:C14H11BrO
- Structure:
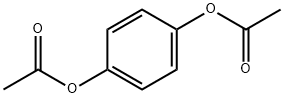
- Chemical Name:1,4-Diacetoxybenzene
- CAS:1205-91-0
- MF:C10H10O4
- Structure:
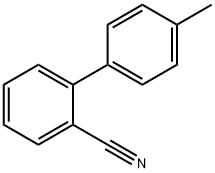
- Chemical Name:4'-Methyl-2-cyanobiphenyl
- CAS:114772-53-1
- MF:C14H11N
- Structure:
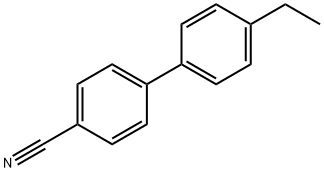
- Chemical Name:4-Cyano-4'-ethylbiphenyl
- CAS:58743-75-2
- MF:C15H13N
- Structure:
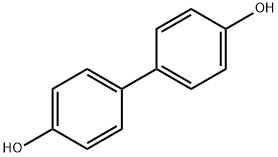
- Chemical Name:4,4'-Biphenol
- CAS:92-88-6
- MF:C12H10O2
- Structure:
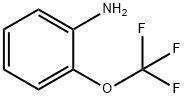
- Chemical Name:2-(Trifluoromethoxy)aniline
- CAS:1535-75-7
- MF:C7H6F3NO
- Structure:
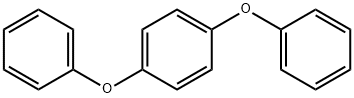
- Chemical Name:1,4-Diphenoxybenzene
- CAS:3061-36-7
- MF:C18H14O2
- Structure:
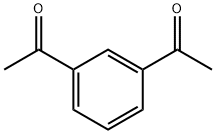
- Chemical Name:1,3-DIACETYLBENZENE
- CAS:6781-42-6
- MF:C10H10O2
- Structure:
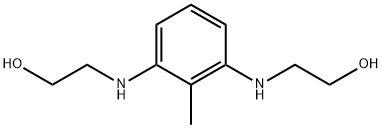
- Chemical Name:Bis-2,6-N,N-(2-hydroxyethyl)diaminotoluene
- CAS:149330-25-6
- MF:C11H18N2O2
- Structure:
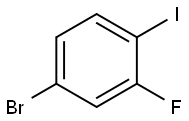
- Chemical Name:4-Bromo-2-fluoro-1-iodobenzene
- CAS:105931-73-5
- MF:C6H3BrFI
- Structure:
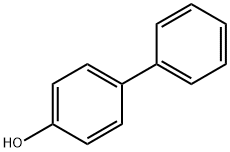
- Chemical Name:4-Phenylphenol
- CAS:92-69-3
- MF:C12H10O
- Structure:
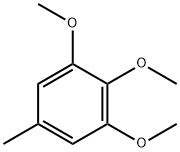
- Chemical Name:3,4,5-Trimethoxytoluene
- CAS:6443-69-2
- MF:C10H14O3
- Structure:
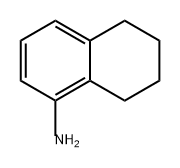
- Chemical Name:5,6,7,8-Tetrahydro-1-naphthylamine
- CAS:2217-41-6
- MF:C10H13N
- Structure:
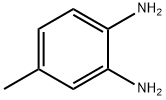
- Chemical Name:3,4-Diaminotoluene
- CAS:496-72-0
- MF:C7H10N2
- Structure:
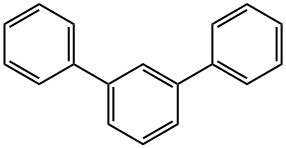
- Chemical Name:1,3-Diphenylbenzene
- CAS:92-06-8
- MF:C18H14
- Structure:
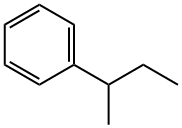
- Chemical Name:SEC-BUTYLBENZENE
- CAS:135-98-8
- MF:C10H14
- Structure:
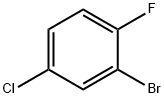
- Chemical Name:2-Bromo-4-chloro-1-fluorobenzene
- CAS:1996-30-1
- MF:C6H3BrClF
- Structure:
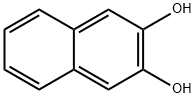
- Chemical Name:2,3-Dihydroxynaphthalene
- CAS:92-44-4
- MF:C10H8O2
- Structure:
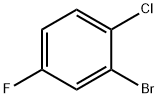
- Chemical Name:2-Bromo-1-chloro-4-fluorobenzene
- CAS:201849-15-2
- MF:C6H3BrClF
- Structure:
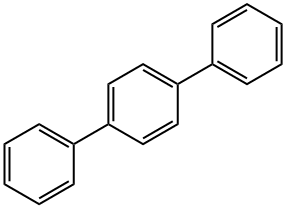
- Chemical Name:p-Terphenyl
- CAS:92-94-4
- MF:C18H14
- Structure:
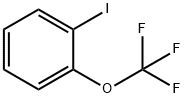
- Chemical Name:1-Iodo-2-(trifluoromethoxy)benzene
- CAS:175278-00-9
- MF:C7H4F3IO
- Structure:
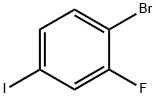
- Chemical Name:1-BROMO-2-FLUORO-4-IODOBENZENE
- CAS:136434-77-0
- MF:C6H3BrFI
- Structure:
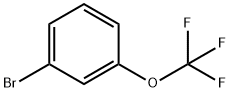
- Chemical Name:3-(Trifluoromethoxy)bromobenzene
- CAS:2252-44-0
- MF:C7H4BrF3O
- Structure:
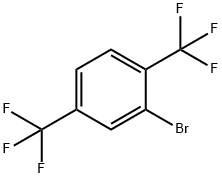
- Chemical Name:2,5-Bis(trifluoromethyl)bromobenzene
- CAS:7617-93-8
- MF:C8H3BrF6
- Structure:
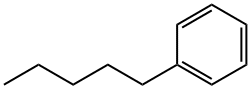
- Chemical Name:Phenylpentane
- CAS:538-68-1
- MF:C11H16
- Structure:
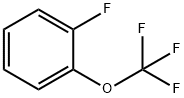
- Chemical Name:2-(Trifluoromethoxy)fluorobenzene
- CAS:2106-18-5
- MF:C7H4F4O
- Structure:
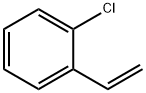
- Chemical Name:2-Chlorostyrene
- CAS:2039-87-4
- MF:C8H7Cl
- Structure:
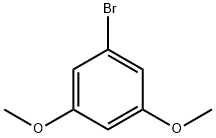
- Chemical Name:1-Bromo-3,5-dimethoxybenzene
- CAS:20469-65-2
- MF:C8H9BrO2
- Structure:
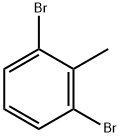
- Chemical Name:2,6-Dibromotoluene
- CAS:69321-60-4
- MF:C7H6Br2
- Structure:
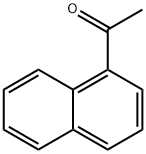
- Chemical Name:1'-Acetonaphthone
- CAS:941-98-0
- MF:C12H10O