Plasticizers
Plasticizers refer to the substance that can enhance the plastic property of polymer compound and polymer materials. In 1868, Hyatt began to take camphor as a plasticizer of cellulose nitrate. The plasticizing effect of the plasticizer is due to the insertion of the plasticizer molecules between the molecular chains of polymers, making the gravity between polymer chains be weakened, namely weaken the aggregation of molecular chains, and increase the movement property, softness of molecular chains, making the plastic property increased.
Based on its compatibility, the plasticizer can be divided into major plasticizers, auxiliary plasticizers and extenders, three types. Ideal plasticizer should have the following properties: good compatibility with the polymer; high plasticization efficiency; light, heat, cold, good weather resistance; low volatility; small migration property; excellent water resistance, oil resistance, and solvent resistance, excellent flexibility in low temperature, flame retardancy, excellent bacterial resistance, excellent electrical insulation, non-toxicity, being odorless, tasteless and colorless, excellent stain resistance as well as being cheap and easy to get. One plasticizer is difficult to simultaneously satisfy all the above-mentioned properties. People commonly use several types in combination. In general, the supplemented plasticizer is always small molecule of high boiling point, low volatility and having good compatibility with high molecular weight polymer.
Plasticizers are substances that can increase the plasticity and fluidity of the polymer and improve the workability and make the material have flexibility. For example, there is a great force between the molecular chains of polyvinyl chloride with the products being hard and lack of flexibility. It can be made into soft PVC product after addition of a plasticizer, expanding the range and types of products.
At the same time, due to the poor thermal stability of PVC, it will decompose and undergo color change after being heated to 130 ~ 140 ℃. Addition of plasticizers can help to reduce the processing temperature and improve the processing performance. Some plasticizers can simultaneously enhance the cold resistance, flame resistance, mildew resistance, antistatic properties, and moisture resistance and so on. There is as many as of thousands of compounds that being able to be taken as plasticizers. According to the chemical structure, it can be classified into phthalic esters, aliphatic dibasic acid esters, phosphate esters, phosphites, fatty acid esters, polyester, epoxy esters, alkyl sulfonic acid phenyl ester, chlorinated plasticizers, polyol esters, trimellitic acid esters and chlorinated compounds.
There are nearly 300 species that have been applied to industrial production. However, there are only 100 kinds that subjecting to wide application, including phthalic acid esters (such as dibutyl phthalate, dioctyl phthalate, etc.) which are used in the largest amount. Commonly used varieties also include adipate, azelate, sebacate, stearate, phosphate esters, and paraffin and so on. Among various kinds of additives of plastic, the production of plasticizer is second to none. More than 80% of plasticizers have been applied to flexible PVC products with the rest being polyvinyl acetate, vinyl chloride - vinyl acetate copolymer, cellulose acetate, and cellulose nitrate.
Although the study history of plasticizing mechanism has been over 40 years, but so far there is still no systematic theory. It is generally thought that the addition of plasticizers weakens the interaction between the polymers, there are two explanations:
① the plasticizing effect of the polar plasticizer on the polar polymers lies in the coupling of the polar groups of them, weakening the polarization effect between polymers.
②The plasticizing effect of non-polar plasticizers on the non-polar polymers can be attributable to isolation effect with the plasticizer molecules being inserted between the polymers and further increasing the spacing between polymers. This method of external supplement of plasticizer is often referred to as external plasticizing. For crystalline polymers or high-polar polymers, if there are no suitable plasticizers, we can introduce side groups or short branched-chain to the polymer chain, weakening the effect between the polymer chains. This method is called internal plasticizing.
- Structure:
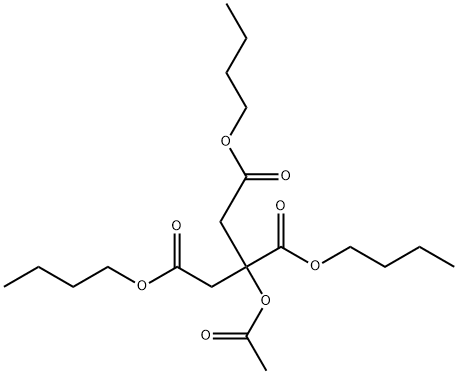
- Chemical Name:Acetyl tributyl citrate
- CAS:77-90-7
- MF:C20H34O8
- Structure:
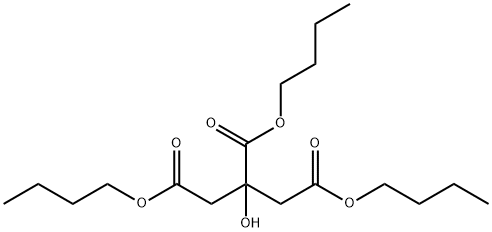
- Chemical Name:Tributyl citrate
- CAS:77-94-1
- MF:C18H32O7
- Structure:
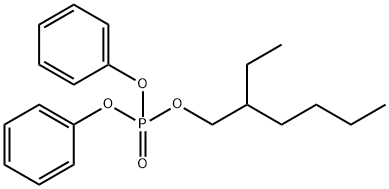
- Chemical Name:2-Ethylhexyl diphenyl phosphate
- CAS:1241-94-7
- MF:C20H27O4P
- Structure:
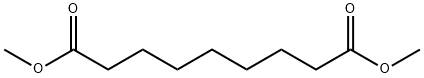
- Chemical Name:Dimethyl azelate
- CAS:1732-10-1
- MF:C11H20O4
- Structure:
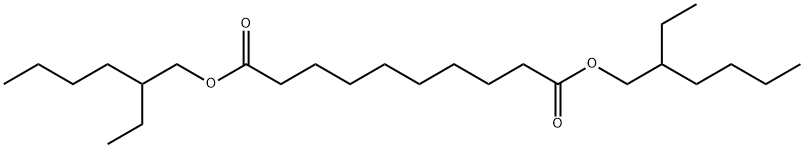
- Chemical Name:Bis(2-ethylhexyl) sebacate
- CAS:122-62-3
- MF:C26H50O4
- Structure:
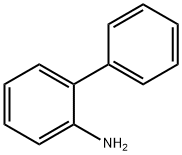
- Chemical Name:2-Aminobiphenyl
- CAS:90-41-5
- MF:C12H11N
- Structure:
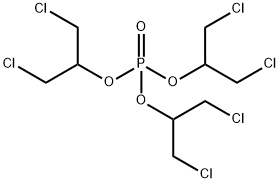
- Chemical Name:Tris(1,3-dichloro-2-propyl)phosphate
- CAS:13674-87-8
- MF:C9H15Cl6O4P
- Structure:
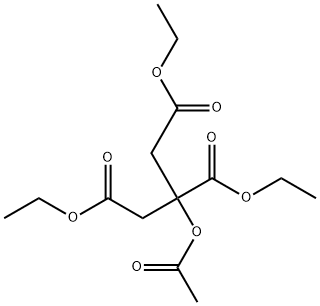
- Chemical Name:Triethyl acetyl citrate
- CAS:77-89-4
- MF:C14H22O8
- Chemical Name:Chlorinated paraffin
- CAS:63449-39-8
- MF:C24H30Cl20C15H14Cl18
- Structure:
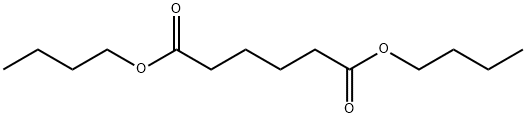
- Chemical Name:Dibutyl adipate
- CAS:105-99-7
- MF:C14H26O4
- Structure:
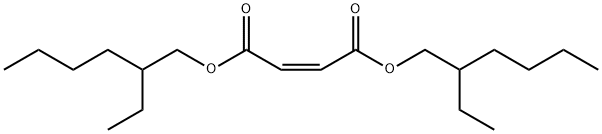
- Chemical Name:Bis(2-ethylhexyl) maleate
- CAS:142-16-5
- MF:C20H36O4
- Structure:
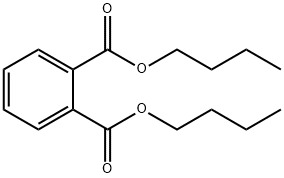
- Chemical Name:Dibutyl phthalate
- CAS:84-74-2
- MF:C16H22O4
- Structure:
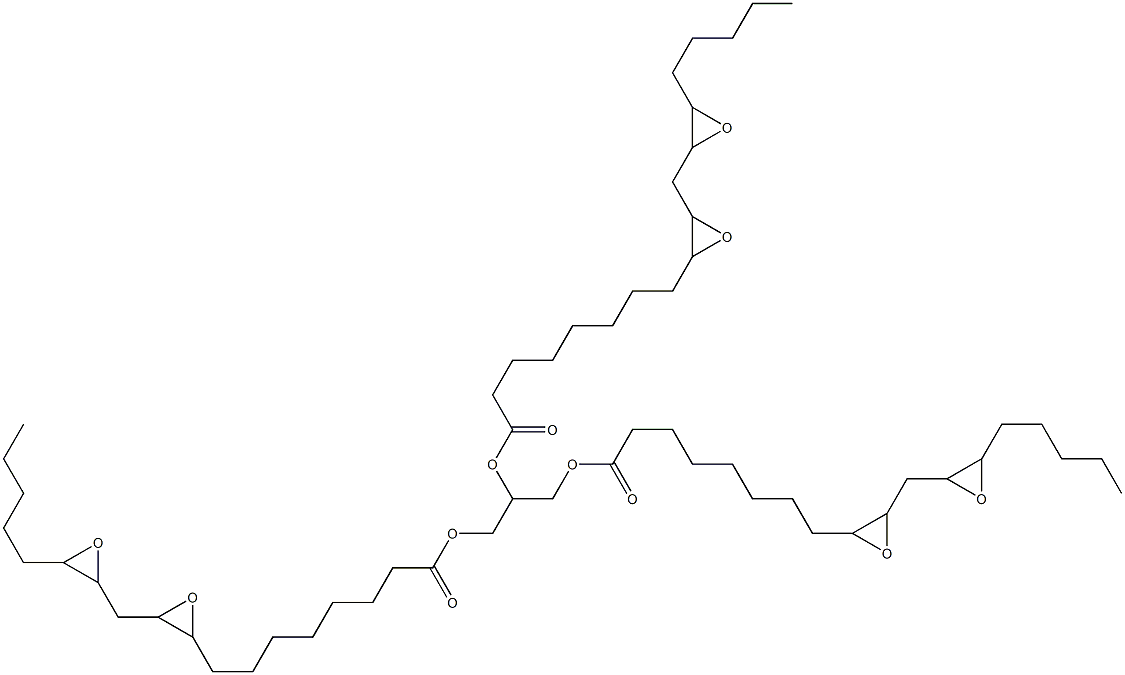
- Chemical Name:Epoxidized soya bean oil
- CAS:8013-07-8
- MF:C57H98O12
- Structure:
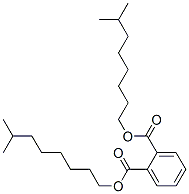
- Chemical Name:Diisononyl phthalate
- CAS:68515-48-0
- MF:C26H42O4
- Structure:
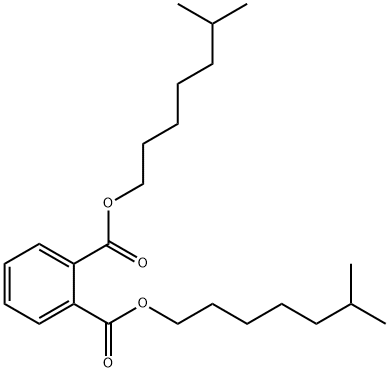
- Chemical Name:Diisooctyl phthalate
- CAS:27554-26-3
- MF:C24H38O4
- Structure:
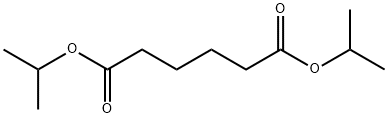
- Chemical Name:Diisopropyl adipate
- CAS:6938-94-9
- MF:C12H22O4
- Structure:
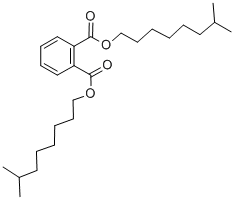
- Chemical Name:Diisononyl phthalate
- CAS:28553-12-0
- MF:C26H42O4
- Structure:
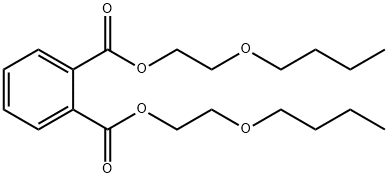
- Chemical Name:BIS(2-N-BUTOXYETHYL)PHTHALATE
- CAS:117-83-9
- MF:C20H30O6
- Structure:
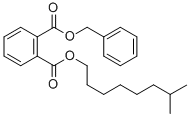
- Chemical Name:BENZYL ISONONYL PHTHALATE
- CAS:126198-74-1
- MF:C24H30O4
- Structure:
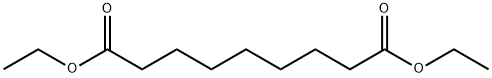
- Chemical Name:DIETHYL AZELATE
- CAS:624-17-9
- MF:C13H24O4
- Structure:
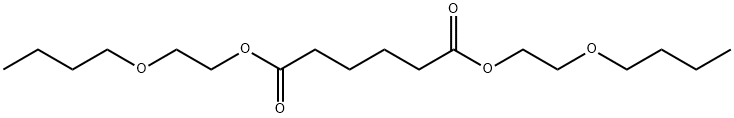
- Chemical Name:BIS(2-BUTOXYETHYL) ADIPATE
- CAS:141-18-4
- MF:C18H34O6
- Structure:
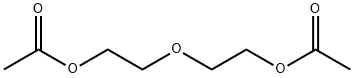
- Chemical Name:Diethyleneglycol diacetate
- CAS:628-68-2
- MF:C8H14O5
- Structure:
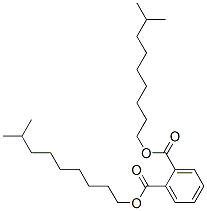
- Chemical Name:1,2-Benzenedicarboxylic acid di-C9-11-branched alkyl esters C10-rich
- CAS:68515-49-1
- MF:C28H46O4
- Chemical Name:CHLOROPARAFFIN C10-C13, 51,5% CL
- CAS:106232-86-4
- MF:C24H44Cl6
- Structure:
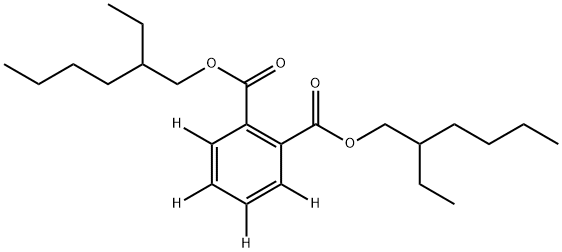
- Chemical Name:BIS(2-ETHYLHEXYL)PHTHALATE (RING-D4)
- CAS:93951-87-2
- MF:C24H38O4
- Structure:

- Chemical Name:2-BUTOXYETHYL OLEATE
- CAS:109-39-7
- MF:C24H46O3
- Structure:
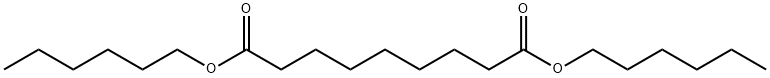
- Chemical Name:DI-N-HEXYL AZELATE
- CAS:109-31-9
- MF:C21H40O4
- Structure:
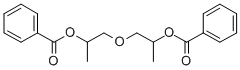
- Chemical Name:Oxydipropyl dibenzoate
- CAS:27138-31-4
- MF:C20H22O5
- Structure:
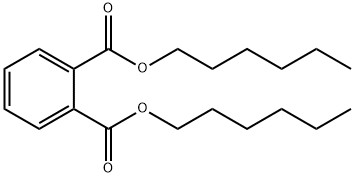
- Chemical Name:DI-N-HEXYL PHTHALATE
- CAS:84-75-3
- MF:C20H30O4
- Structure:
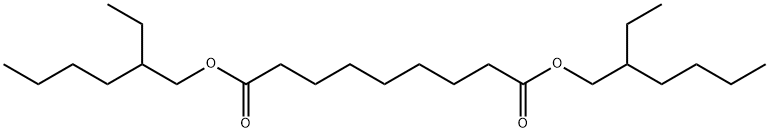
- Chemical Name:AZELAIC ACID DI(2-ETHYLHEXYL) ESTER
- CAS:103-24-2
- MF:C25H48O4
- Structure:
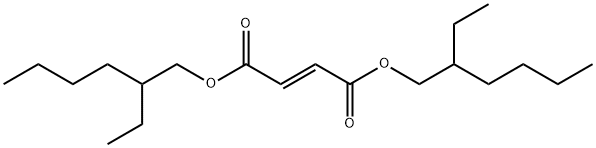
- Chemical Name:DIOCTYL FUMARATE
- CAS:141-02-6
- MF:C20H36O4
- Structure:
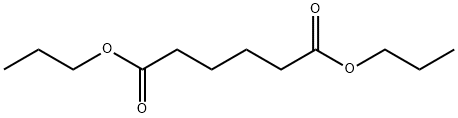
- Chemical Name:Dipropyl adipate
- CAS:106-19-4
- MF:C12H22O4
- Structure:
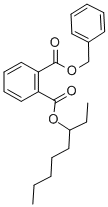
- Chemical Name:BENZYL 2-ETHYLHEXYL PHTHALATE
- CAS:27215-22-1
- MF:C23H28O4
- Structure:

- Chemical Name:HEXYL ACETATE
- CAS:88230-35-7
- MF:C8H16O2
- Structure:
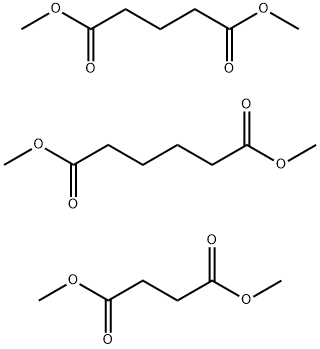
- Chemical Name:DIBASIC ESTER
- CAS:95481-62-2
- MF:C21H36O12
- Structure:
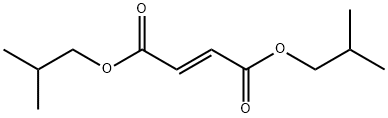
- Chemical Name:Diisobutyl fumarate
- CAS:7283-69-4
- MF:C12H20O4
- Structure:
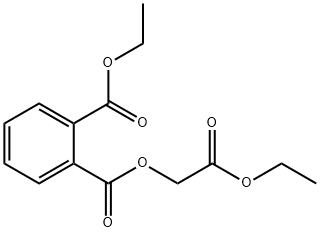
- Chemical Name:ETHYL PHTHALYL ETHYL GLYCOLATE
- CAS:84-72-0
- MF:C14H16O6
- Chemical Name:Chlorinated rubber
- CAS:9006-03-5
- MF:[C10H11Cl7]n
- Chemical Name:Chlorinated Paraffin-70
- CAS:
- MF:
- Structure:
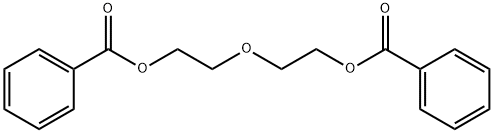
- Chemical Name:Diethylene glycol dibenzoate
- CAS:120-55-8
- MF:C18H18O5
- Structure:
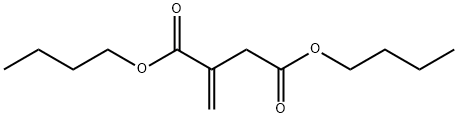
- Chemical Name:DIBUTYL ITACONATE
- CAS:2155-60-4
- MF:C13H22O4
- Structure:
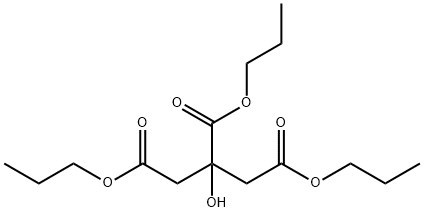
- Chemical Name:CITRIC ACID TRI-N-PROPYL ESTER
- CAS:1587-21-9
- MF:C15H26O7
- Structure:
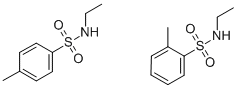
- Chemical Name:Plasticizer 8
- CAS:26914-52-3
- MF:C9H15NO2S
- Structure:
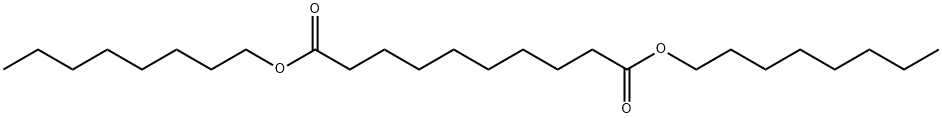
- Chemical Name:SEBACIC ACID DI-N-OCTYL ESTER
- CAS:2432-87-3
- MF:C26H50O4
- Structure:
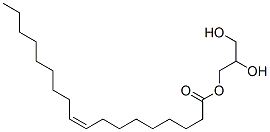
- Chemical Name:Glyceryl monooleate
- CAS:25496-72-4
- MF:C21H40O4
- Chemical Name:cheMlok 205
- CAS:
- MF:
- Structure:
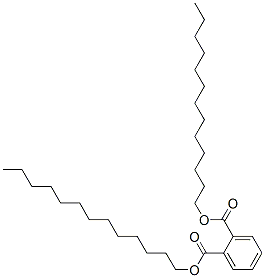
- Chemical Name:DITRIDECYL PHTHALATE
- CAS:75359-31-8
- MF:C34H58O4
- Structure:
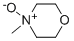
- Chemical Name:4-Methylmorpholine N-oxide monohydrate
- CAS:70187-32-5
- MF:C5H11NO2
- Structure:
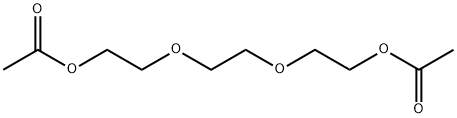
- Chemical Name:Triethylene glycol diacetate
- CAS:111-21-7
- MF:C10H18O6
- Structure:
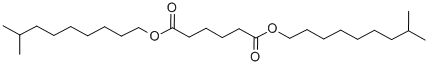
- Chemical Name:DIISODECYL ADIPATE
- CAS:27178-16-1
- MF:C26H50O4
- Structure:
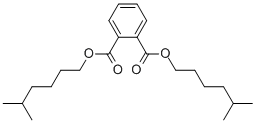
- Chemical Name:DIISOHEPTYL PHTHALATE
- CAS:41451-28-9
- MF:C22H34O4
- Structure:
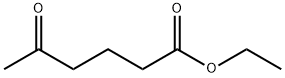
- Chemical Name:Ethyl 4-acetylbutyrate
- CAS:13984-57-1
- MF:C8H14O3
- Structure:
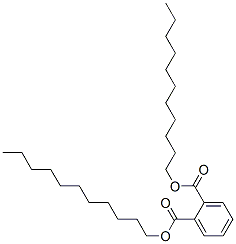
- Chemical Name:DIUNDECYL PHTHALATE
- CAS:96507-86-7
- MF:C30H50O4
- Structure:
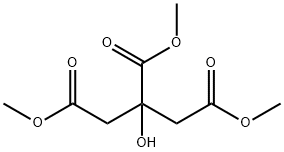
- Chemical Name:TRIMETHYL CITRATE
- CAS:1587-20-8
- MF:C9H14O7
- Structure:
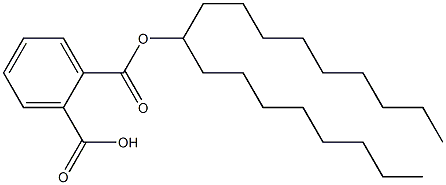
- Chemical Name:Octyl-decyl phthalate
- CAS:
- MF:C26H42O4
- Structure:

- Chemical Name:DIISOHEPTYL PHTHALATE
- CAS:71888-89-6
- MF:C22H34O4
- Structure:
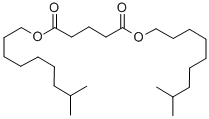
- Chemical Name:DIISODECYL GLUTARATE
- CAS:29733-18-4
- MF:C25H48O4
- Structure:
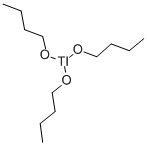
- Chemical Name:BUTYL TALLATE
- CAS:67762-63-4
- MF:C12H27O3Tl
- Structure:

- Chemical Name:tetraoctyl pyromellitate
- CAS:
- MF:C42H70O8
- Structure:
- Chemical Name:POLY(PROPYLENE GLYCOL) DIBENZOATE
- CAS:72245-46-6
- MF:C9H12O4
- Structure:
- Chemical Name:POLY(ETHYLENE-CO-VINYL ACETATE-CO-CARBON MONOXIDE)
- CAS:26337-35-9
- MF:C7H10O3
- Structure:
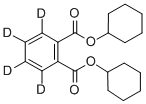
- Chemical Name:DICYCLOHEXYL PHTHALATE-3,4,5,6-D4
- CAS:358731-25-6
- MF:C20H22D4O4
- Structure:
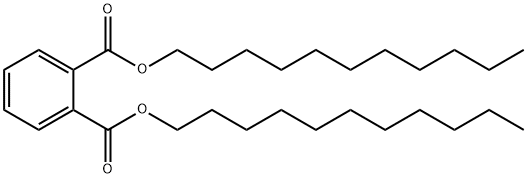
- Chemical Name:Diundecyl phthalate
- CAS:3648-20-2
- MF:C30H50O4
- Structure:
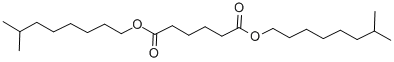
- Chemical Name:Diisononyl adipate
- CAS:33703-08-1
- MF:C24H46O4
- Structure:
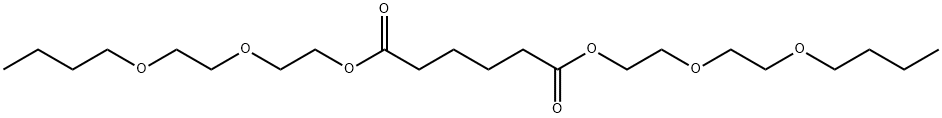
- Chemical Name:BIS[2-(2-BUTOXYETHOXY)ETHYL] ADIPATE
- CAS:141-17-3
- MF:C22H42O8
- Structure:
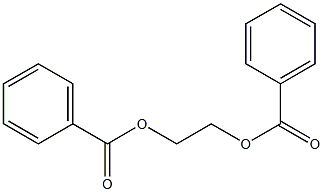
- Chemical Name:POLY(ETHYLENE GLYCOL) DIBENZOATE
- CAS:9004-86-8
- MF:C16H14O4
- Structure:
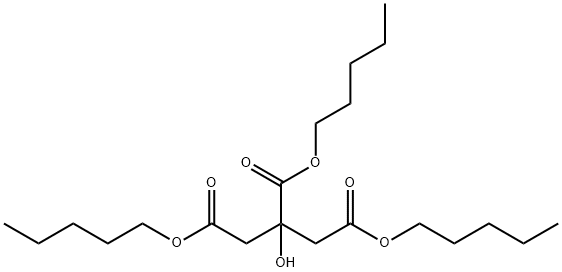
- Chemical Name:TRI-N-AMYL CITRATE
- CAS:70289-34-8
- MF:C21H38O7
- Chemical Name:wax synethesis
- CAS:
- MF:
- Structure:
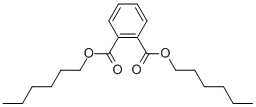
- Chemical Name:DI-N-HEXYL PHTHALATE
- CAS:68515-50-4
- MF:C20H30O4
- Structure:
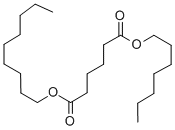
- Chemical Name:ADIPIC ACID HEPTYLNONYL ESTER
- CAS:68515-75-3
- MF:C22H42O4
- Structure:
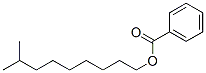
- Chemical Name:ISODECYL BENZOATE
- CAS:131298-44-7
- MF:C17H26O2
- Structure:
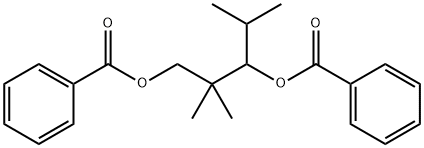
- Chemical Name:2,2,4-TRIMETHYL-1,3-PENTANEDIOL DIBENZOATE
- CAS:68052-23-3
- MF:C22H26O4
- Chemical Name:DI-N-ALKYL ADIPATE
- CAS:
- MF: