Aliphatic dicarboxylic acid esters
Ester spices synthesized from carboxylic acids and alcohols are a very important category of spices. According to the raw materials used in the synthesis, they are roughly divided into: aliphatic carboxylic esters, unsaturation carboxylic esters such as containing alkene and alkyne, aromatic carboxylic esters and other esters. One characteristic of ester spices is that carboxylic esters can be considered as the product of carboxylic acid after its hydroxyl groups are replaced by alkoxy groups. Because hydroxyl groups can be substituted with one or more kinds of alkoxy groups, the product can be monoester, diester or multiesters. Furthermore, an alcohol can also have several hydroxyl groups, which can react with one or more kinds of carboxyls to form a monocarboxylic ester or a multicarboxylic ester. The aroma and its type, intensity and characteristics are related to the structure of esters. An ester product of a lower carboxylic acid and a lower alcohol is generally a volatile liquid, with the aroma of flower, fruit and grass. An ester product of a lower carboxylic acid and a lower terpene alcohol has a flower aroma. Most esters with aryl groups have flower aroma. Ester products of aromatic carboxylic acids and aromatic alcohols generally have a not strong but lasting aroma with a higher boiling point. The widely existed ester spices can be found in the roots, stems, leaves, fruits, seeds, bark, flowers and other parts of plants and also found in some animal secretions. More ester spices are artificially synthesized. Synthetic esters are widely used for their easy synthetic procedures, wide source of raw materials, wide range of flavors and lower cost.
Aliphatic compounds, also known as open-chain compounds belong to one of the basic types of organic compounds. Carbon atoms of these compounds form a chain structure in their molecules. According to the structure and nature of the open-chain they can be divided into saturated and unsaturated aliphatic hydrocarbons. The former only have single bonds between carbon atoms, such as ethane and ethanol; the others such as ethylene and acetylene, have double and triple bonds between carbon atoms. Paraffinic hydrocarbons, alkenes and alkynes and their derivatives all belong to such compounds. Carbon chain structure without branches is called unbranched chain and the branch part is called side chain. Such compounds are abound in nature and industry, e.g. paraffins in swamps, olefins in forests and alkyl lead in automobile exhaust.
- Structure:
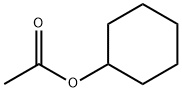
- Chemical Name:Cyclohexyl acetate
- CAS:622-45-7
- MF:C8H14O2
- Structure:
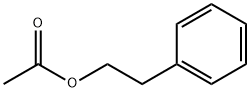
- Chemical Name:Phenethyl acetate
- CAS:103-45-7
- MF:C10H12O2
- Structure:
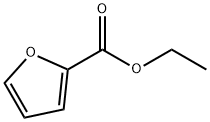
- Chemical Name:Ethyl 2-furoate
- CAS:614-99-3
- MF:C7H8O3
- Structure:
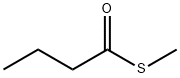
- Chemical Name:Methyl thiobutyrate
- CAS:2432-51-1
- MF:C5H10OS
- Structure:
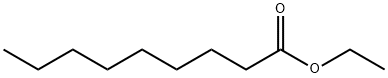
- Chemical Name:Ethyl nonanoate
- CAS:123-29-5
- MF:C11H22O2
- Structure:
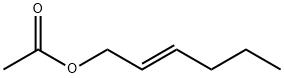
- Chemical Name:trans-2-Hexenyl acetate
- CAS:2497-18-9
- MF:C8H14O2
- Structure:
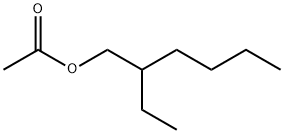
- Chemical Name:2-Ethylhexyl acetate
- CAS:103-09-3
- MF:C10H20O2
- Structure:

- Chemical Name:HEXYL FORMATE
- CAS:629-33-4
- MF:C7H14O2
- Structure:
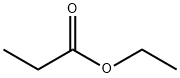
- Chemical Name:Ethyl propionate
- CAS:105-37-3
- MF:C5H10O2
- Structure:
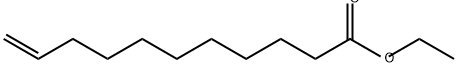
- Chemical Name:ETHYL 10-UNDECENOATE
- CAS:692-86-4
- MF:C13H24O2
- Structure:
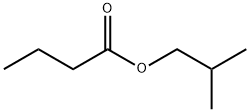
- Chemical Name:Isobutyl butyrate
- CAS:539-90-2
- MF:C8H16O2
- Structure:
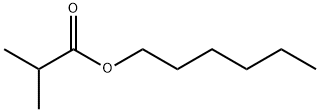
- Chemical Name:Hexyl isobutyrate
- CAS:2349-07-7
- MF:C10H20O2
- Structure:
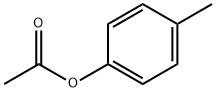
- Chemical Name:P-TOLYL ACETATE
- CAS:140-39-6
- MF:C9H10O2
- Structure:
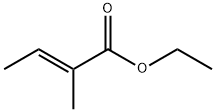
- Chemical Name:Ethyl tiglate
- CAS:5837-78-5
- MF:C7H12O2
- Structure:
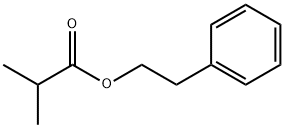
- Chemical Name:Phenethyl isobutyrate
- CAS:103-48-0
- MF:C12H16O2
- Structure:
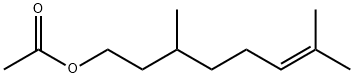
- Chemical Name:Citronellyl acetate
- CAS:150-84-5
- MF:C12H22O2
- Structure:
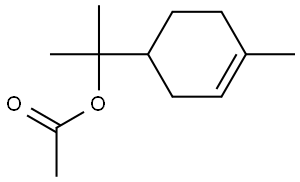
- Chemical Name:Terpinyl acetate
- CAS:80-26-2
- MF:C12H20O2
- Structure:
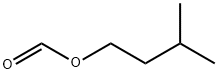
- Chemical Name:Isopentyl formate
- CAS:110-45-2
- MF:C6H12O2
- Structure:
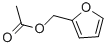
- Chemical Name:Furfuryl acetate
- CAS:623-17-6
- MF:C7H8O3
- Structure:
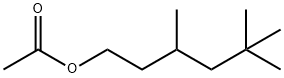
- Chemical Name:3,5,5-TRIMETHYLHEXYL ACETATE
- CAS:58430-94-7
- MF:C11H22O2
- Structure:
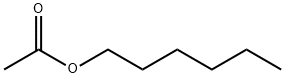
- Chemical Name:Hexyl acetate
- CAS:142-92-7
- MF:C8H16O2
- Structure:
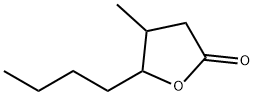
- Chemical Name:Whiskey lactone
- CAS:39212-23-2
- MF:C9H16O2
- Structure:
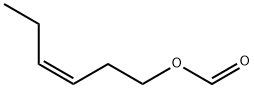
- Chemical Name:cis-3-Hexenyl formate
- CAS:33467-73-1
- MF:C7H12O2
- Structure:
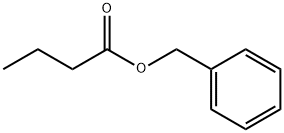
- Chemical Name:Benzyl butyrate
- CAS:103-37-7
- MF:C11H14O2
- Structure:
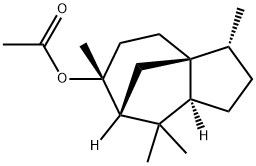
- Chemical Name:Cedryl acetate
- CAS:77-54-3
- MF:C17H28O2
- Structure:
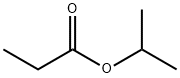
- Chemical Name:ISOPROPYL PROPIONATE
- CAS:637-78-5
- MF:C6H12O2
- Structure:
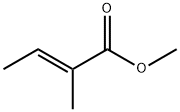
- Chemical Name:Methyl tiglate
- CAS:6622-76-0
- MF:C6H10O2
- Structure:
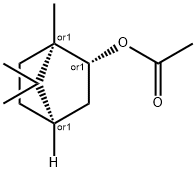
- Chemical Name:Isobornyl acetate
- CAS:125-12-2
- MF:C12H20O2
- Structure:
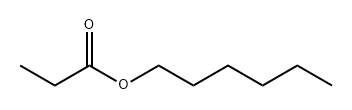
- Chemical Name:Hexyl propionate
- CAS:2445-76-3
- MF:C9H18O2
- Structure:
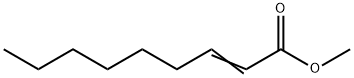
- Chemical Name:Methyl trans-2-nonenoate
- CAS:111-79-5
- MF:C10H18O2
- Structure:
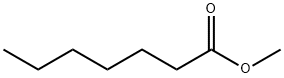
- Chemical Name:Methyl heptanoate
- CAS:106-73-0
- MF:C8H16O2
- Structure:
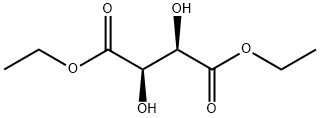
- Chemical Name:L(+)-Diethyl L-tartrate
- CAS:87-91-2
- MF:C8H14O6
- Structure:
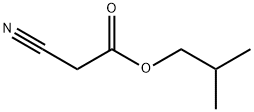
- Chemical Name:Isobutyl cyanoacetate
- CAS:13361-31-4
- MF:C7H11NO2
- Structure:
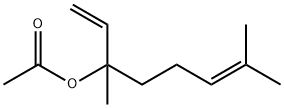
- Chemical Name:Linalyl acetate
- CAS:115-95-7
- MF:C12H20O2
- Structure:
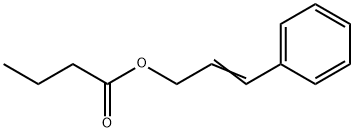
- Chemical Name:FEMA 2296
- CAS:103-61-7
- MF:C13H16O2
- Structure:
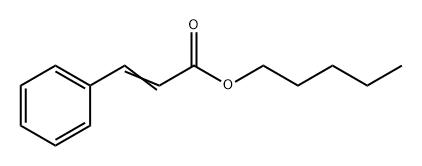
- Chemical Name:Pentyl cinnamate
- CAS:3487-99-8
- MF:C14H18O2
- Structure:
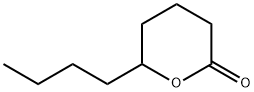
- Chemical Name:delta-Nonalactone
- CAS:3301-94-8
- MF:C9H16O2
- Structure:
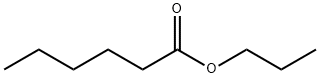
- Chemical Name:Caproic acid propyl ester
- CAS:626-77-7
- MF:C9H18O2
- Structure:
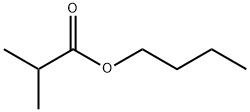
- Chemical Name:Butyl isobutyrate
- CAS:97-87-0
- MF:C8H16O2
- Structure:
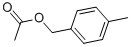
- Chemical Name:4-Methylbenzyl acetate
- CAS:2216-45-7
- MF:C10H12O2
- Structure:
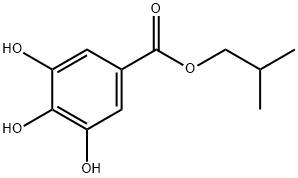
- Chemical Name:ISOBUTYL GALLATE
- CAS:3856-05-1
- MF:C11H14O5
- Structure:
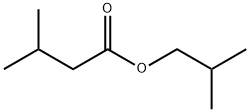
- Chemical Name:Isobutyl isovalerate
- CAS:589-59-3
- MF:C9H18O2
- Structure:
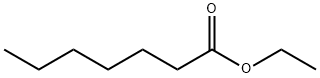
- Chemical Name:Ethyl heptanoate
- CAS:106-30-9
- MF:C9H18O2
- Structure:
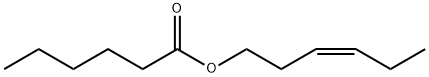
- Chemical Name:cis-3-Hexenyl hexanoate
- CAS:31501-11-8
- MF:C12H22O2
- Structure:
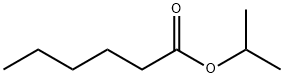
- Chemical Name:N-CAPROIC ACID ISOPROPYL ESTER
- CAS:2311-46-8
- MF:C9H18O2
- Structure:
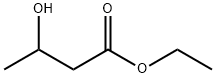
- Chemical Name:Ethyl 3-hydroxybutyrate
- CAS:5405-41-4
- MF:C6H12O3
- Structure:
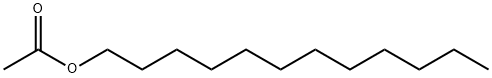
- Chemical Name:DODECYL ACETATE
- CAS:112-66-3
- MF:C14H28O2
- Structure:
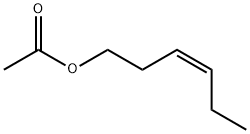
- Chemical Name:cis-3-Hexenyl Acetate
- CAS:3681-71-8
- MF:C8H14O2
- Structure:
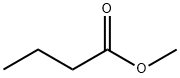
- Chemical Name:Methyl butyrate
- CAS:623-42-7
- MF:C5H10O2
- Structure:
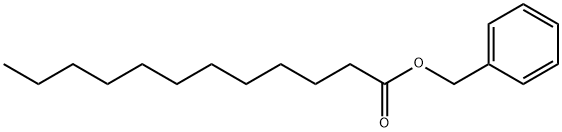
- Chemical Name:BENZYL LAURATE
- CAS:140-25-0
- MF:C19H30O2
- Structure:
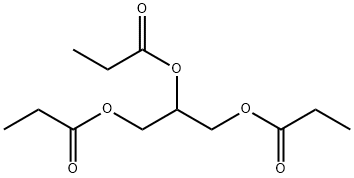
- Chemical Name:Tripropionin
- CAS:139-45-7
- MF:C12H20O6
- Structure:
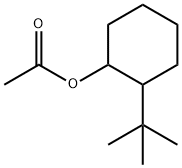
- Chemical Name:O-TERT-BUTYLCYCLOHEXYL ACETATE
- CAS:88-41-5
- MF:C12H22O2
- Structure:
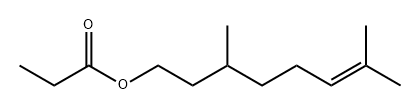
- Chemical Name:CITRONELLYL PROPIONATE
- CAS:141-14-0
- MF:C13H24O2
- Structure:
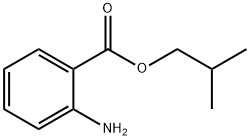
- Chemical Name:Isobutyl anthranilate
- CAS:7779-77-3
- MF:C11H15NO2
- Structure:
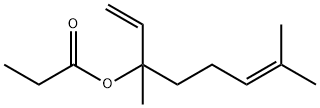
- Chemical Name:LINALYL PROPIONATE
- CAS:144-39-8
- MF:C13H22O2
- Structure:
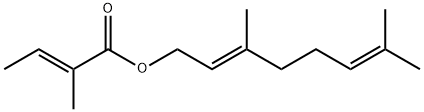
- Chemical Name:Geranyl tiglate
- CAS:7785-33-3
- MF:C15H24O2
- Structure:
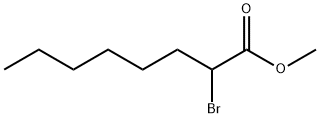
- Chemical Name:METHYL 2-BROMOOCTANOATE
- CAS:5445-22-7
- MF:C9H17BrO2
- Structure:
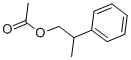
- Chemical Name:2-Phenylpropyl acetate
- CAS:10402-52-5
- MF:C11H14O2
- Structure:
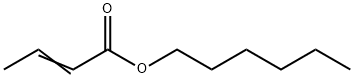
- Chemical Name:FEMA 3354
- CAS:19089-92-0
- MF:C10H18O2
- Structure:
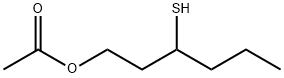
- Chemical Name:3-Mercaptohexyl acetate
- CAS:136954-20-6
- MF:C8H16O2S
- Structure:
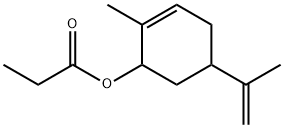
- Chemical Name:(-)-CARVYL PROPIONATE
- CAS:97-45-0
- MF:C13H20O2
- Structure:
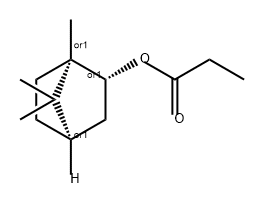
- Chemical Name:Isobornyl propanoate
- CAS:2756-56-1
- MF:C13H22O2
- Structure:
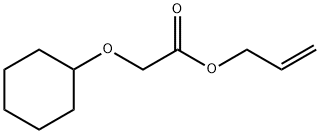
- Chemical Name:Allyl cyclohexyloxyacetate
- CAS:68901-15-5
- MF:C11H18O3
- Structure:
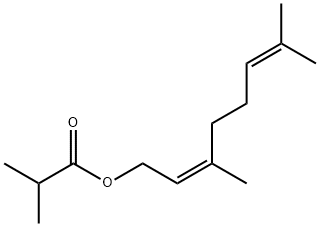
- Chemical Name:NERYL ISOBUTYRATE
- CAS:2345-24-6
- MF:C14H24O2
- Structure:

- Chemical Name:OCTYL FORMATE
- CAS:112-32-3
- MF:C9H18O2
- Structure:
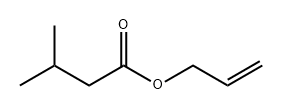
- Chemical Name:ALLYL ISOVALERATE
- CAS:2835-39-4
- MF:C8H14O2
- Structure:
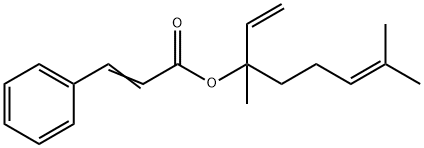
- Chemical Name:LINALYL CINNAMATE
- CAS:78-37-5
- MF:C19H24O2
- Structure:
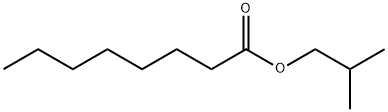
- Chemical Name:N-CAPRYLIC ACID ISOBUTYL ESTER
- CAS:5461-06-3
- MF:C12H24O2
- Structure:
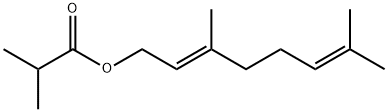
- Chemical Name:GERANYL ISOBUTYRATE
- CAS:2345-26-8
- MF:C14H24O2
- Structure:
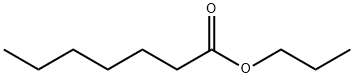
- Chemical Name:Propyl heptanoate
- CAS:7778-87-2
- MF:C10H20O2
- Structure:
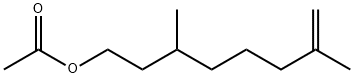
- Chemical Name:RHODINYL ACETATE
- CAS:141-11-7
- MF:C12H22O2
- Structure:
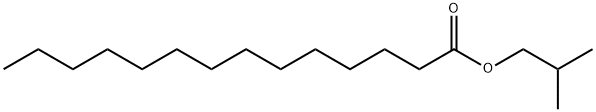
- Chemical Name:MYRISTIC ACID ISOBUTYL ESTER
- CAS:25263-97-2
- MF:C18H36O2
- Structure:
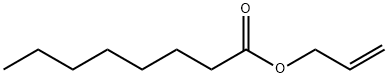
- Chemical Name:ALLYL CAPRYLATE
- CAS:4230-97-1
- MF:C11H20O2
- Structure:
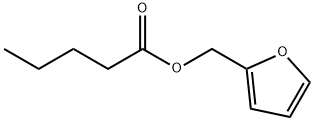
- Chemical Name:Furfuryl pentanoate
- CAS:36701-01-6
- MF:C10H14O3
- Structure:
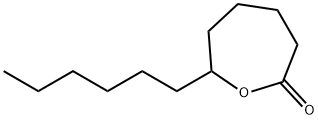
- Chemical Name:DELTA-DODECANOLACTONE
- CAS:16429-21-3
- MF:C12H22O2
- Structure:
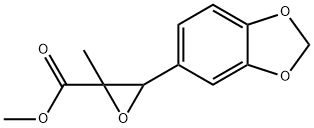
- Chemical Name:Pmk glycidate
- CAS:13605-48-6
- MF:C12H12O5
- Structure:
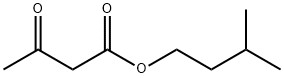
- Chemical Name:ACETOACETIC ACID ISOAMYL ESTER
- CAS:2308-18-1
- MF:C9H16O3
- Structure:
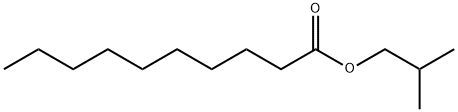
- Chemical Name:ISOBUTYL DECANOATE
- CAS:30673-38-2
- MF:C14H28O2
- Structure:
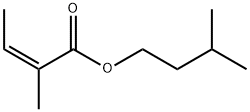
- Chemical Name:ANGELIC ACID ISOAMYL ESTER
- CAS:10482-55-0
- MF:C10H18O2
- Structure:
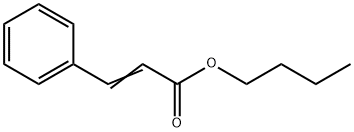
- Chemical Name:N-BUTYL CINNAMATE
- CAS:538-65-8
- MF:C13H16O2
- Structure:
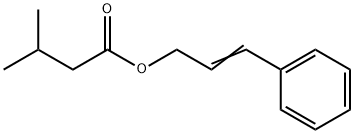
- Chemical Name:FEMA 2302
- CAS:140-27-2
- MF:C14H18O2
- Structure:
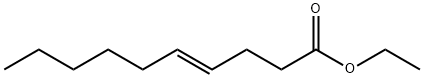
- Chemical Name:ETHYL TRANS-4-DECENOATE
- CAS:76649-16-6
- MF:C12H22O2
- Structure:
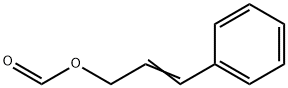
- Chemical Name:CINNAMYL FORMATE
- CAS:104-65-4
- MF:C10H10O2
- Structure:
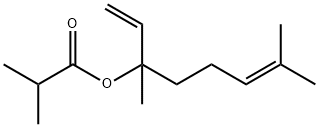
- Chemical Name:LINALYL ISOBUTYRATE
- CAS:78-35-3
- MF:C14H24O2
- Structure:
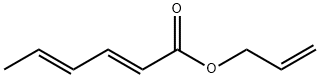
- Chemical Name:ALLYL SORBATE
- CAS:7493-75-6
- MF:C9H12O2
- Structure:
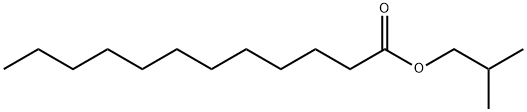
- Chemical Name:LAURIC ACID ISOBUTYL ESTER
- CAS:37811-72-6
- MF:C16H32O2
- Structure:
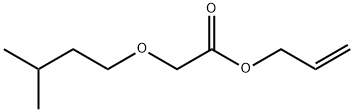
- Chemical Name:Allyl (3-methylbutoxy)acetate
- CAS:67634-00-8
- MF:C10H18O3
- Structure:
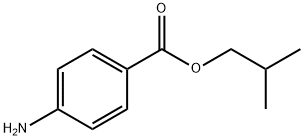
- Chemical Name:4-AMINOBENZOIC ACID ISOBUTYL ESTER
- CAS:94-14-4
- MF:C11H15NO2
- Structure:
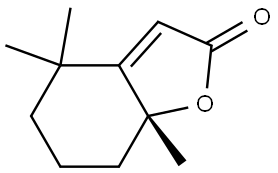
- Chemical Name:Dihydroactinidiolide
- CAS:17092-92-1
- MF:C11H16O2
- Structure:
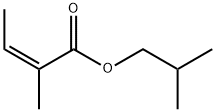
- Chemical Name:ISOBUTYL ANGELATE
- CAS:7779-81-9
- MF:C9H16O2
- Structure:
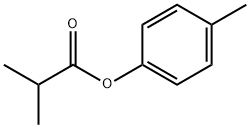
- Chemical Name:P-TOLYL ISOBUTYRATE
- CAS:103-93-5
- MF:C11H14O2
- Structure:
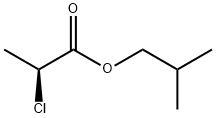
- Chemical Name:(S)-Isobutyl-2-chloropropanoate
- CAS:83261-15-8
- MF:C7H13ClO2
- Structure:
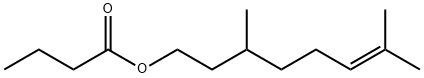
- Chemical Name:CITRONELLYL BUTYRATE
- CAS:141-16-2
- MF:C14H26O2
- Structure:
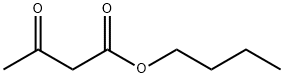
- Chemical Name:ACETOACETIC ACID N-BUTYL ESTER
- CAS:591-60-6
- MF:C8H14O3
- Structure:
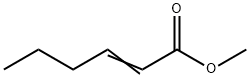
- Chemical Name:Methyl 2-hexenoate
- CAS:2396-77-2
- MF:C7H12O2
- Structure:
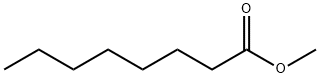
- Chemical Name:Caprylic acid methyl ester
- CAS:111-11-5
- MF:C9H18O2
- Structure:
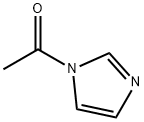
- Chemical Name:1-Acetylimidazole
- CAS:2466-76-4
- MF:C5H6N2O
- Structure:
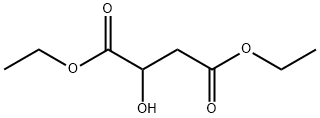
- Chemical Name:Diethyl malate
- CAS:7554-12-3
- MF:C8H14O5
- Structure:
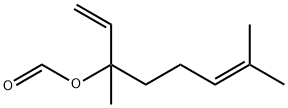
- Chemical Name:LINALYL FORMATE
- CAS:115-99-1
- MF:C11H18O2
- Structure:

- Chemical Name:Isooctyl acetate
- CAS:31565-19-2
- MF:C10H20O2