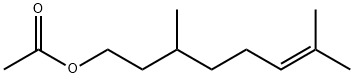
Citronellyl acetate has a fresh, fruity odor reminiscent of rose and a pungent taste at the beginning, turning to sweet,
apricot-like taste afterward.
Citronellyl Acetate occurs in many
essential oils either as one of its optical isomers or as the racemate. The odor of
racemic citronellyl acetate differs little from that of the optical isomers. Racemic
citronellyl acetate is a liquid with a fresh, fruity rose odor. It is often used as a fragrance,
for example, for rose, lavender, and geranium notes as well as for eau de
cologne with citrus nuances. Since it is relatively stable to alkali, it can be used in
soaps and detergents. Citrus flavors acquire specific character through the addition
of citronellyl acetate; it is also used to round off other fruit flavors.
Reported in the oil of citronella, Chamaecyparis lawsoniana Parl, orange juice, lemon juice, lemon and grapefruit
peel oil, swangi (Citrus hystrix D.C.), ginger, tarragon, myrtle leaf, nutmeg, Cympogon citralis oil and beer.
Citronellyl acetate is used as a reactant, and oxidized by ruthenium tetroxide catalyst in presence of acetonitrile.
ChEBI: A monoterpenoid that is the acetate ester of citronellol. It has been isolated from Citrus hystrix.
By esterification of citronellol with acetic acid or acetic anhydride.
Taste characteristics at 30 ppm: floral, green, fruity, sweet, citrus and waxy character.
Flammability and Explosibility
Non flammable
Mildly toxic by ingestion. A human skin irritant. See also ESTERS. Combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes.
By direct acetylation of citronellol (natural or synthetic); its physical–chemical characteristics vary, depending on the
quality of the starting alcohol.