Monosaccharide
Monosaccharide is the polyhydroxy aldehyde or ketone comprising 3 to 7 carbon atoms in combination with hydrogen and oxygen atoms. The simplest monosaccharide is triose monosaccharide containing three carbon atoms. With the number increase of carbon atoms , there are tetrose, pentose and hexose and the like. The most important pentose in human body is ribose and deoxyribose, which form the RNA and DNA. The three most important monosaccharides are glucose, fructose and galactose, of which C6H12O6 is the common chemical formula. Monosaccharide structure has aldehyde or ketone group: glucose and galactose contain aldehyde group, and fructose contains ketone group. Due to the existence of aldehyde and ketone group, monosaccharide has reducibility and can be used to check whether there is urine or blood sugar. Monosaccharide contains multiple hydroxyl groups and can be used for esterification, glycosidation, deoxygenation, dehydration and amination reaction. Monosaccharide is the basic unit of many sugars, and the carbohydrate foods can be digested into monosaccharides, which are absorbed in the small intestine. Different monosaccharides have different absorption rates: 100 of glucose, 110 of galactose, 43 of fructose, 19 of mannose, 15 of xylulose, 9 of arabinose.
Monosaccharides are the basic components of marine life and carbohydrates in sea water and marine sediments. The major monosaccharides in marine plankton include (as usual abundance in the order listed) galactose, glucose, mannose, ribose, xylose, fucose, rhamnose and arabinose. The present forms of sugars in vivo are mainly polysaccharide and combined sugar. These monosaccharides can also be commonly found in seawater and sediments. Fructose lacks a big biological source in the ocean, but can be converted from glucose by epimerization in seawater.
The concentration range of individual monosaccharides in seawater is 0-20 μg/ L, and 10 mg carbon / L generally represents the total concentration of free monosaccharides. The total free monosaccharide content in seawater can be measured by the method of METH. The glucose content can be directly measured via enzymatic reaction or bioassay. The simultaneous determination of all individual monosaccharides in seawater sample are commonly carried out through chromatography. Since monosaccharide is non-volatile compounds, it must be processed via derivatization when used for gas chromatography, in which the same monosaccharide appears in different forms of anomer and gives complex peaks. Liquid chromatography have more applications, such as the ethanol / water partition chromatography on anion exchange resin and the ion exchange chromatography in which boric acid complexes formed on the borate anion exchange resin.
- Structure:
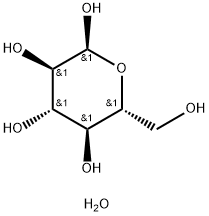
- Chemical Name:D(+)-GLUCOSE MONOHYDRATE
- CAS:14431-43-7
- MF:C6H14O7
- Structure:
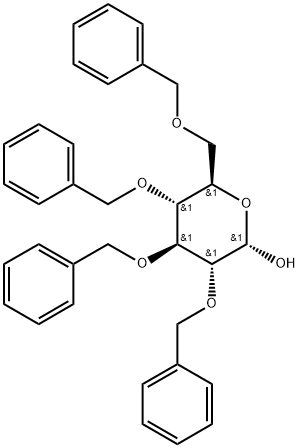
- Chemical Name:2,3,4,6-TETRA-O-BENZYL-ALPHA-D-GLUCOPYRANOSE
- CAS:6564-72-3
- MF:C34H36O6
- Structure:
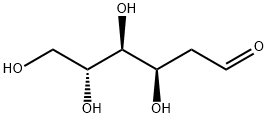
- Chemical Name:2-Deoxy-D-glucose
- CAS:154-17-6
- MF:C6H12O5
- Structure:
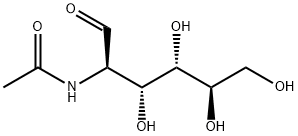
- Chemical Name:N-Acetyl-D-Glucosamine
- CAS:7512-17-6
- MF:C8H15NO6
- Structure:
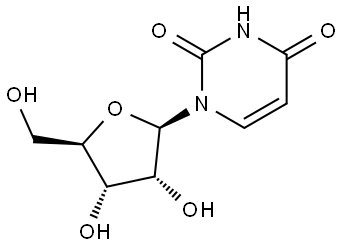
- Chemical Name:Uridine
- CAS:58-96-8
- MF:C9H12N2O6
- Structure:
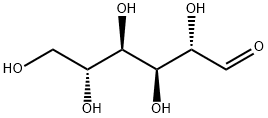
- Chemical Name:D-Mannose
- CAS:3458-28-4
- MF:C6H12O6
- Structure:
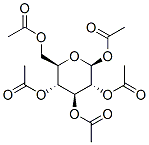
- Chemical Name:β-D-Glucose pentaacetate
- CAS:604-69-3
- MF:C16H22O11
- Structure:
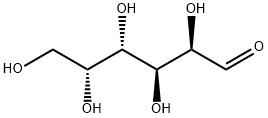
- Chemical Name:D-Galactose
- CAS:59-23-4
- MF:C6H12O6
- Structure:
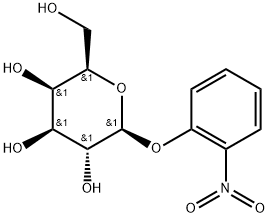
- Chemical Name:2-Nitrophenyl-beta-D-galactopyranoside
- CAS:369-07-3
- MF:C12H15NO8
- Structure:
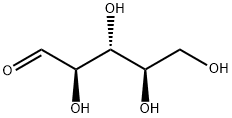
- Chemical Name:D-Ribose
- CAS:50-69-1
- MF:C5H10O5
- Structure:
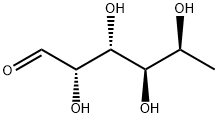
- Chemical Name:L-Fucose
- CAS:2438-80-4
- MF:C6H12O5
- Structure:
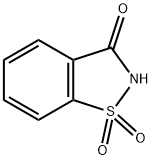
- Chemical Name:Saccharin
- CAS:81-07-2
- MF:C7H5NO3S
- Structure:
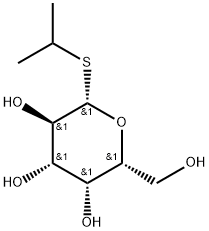
- Chemical Name:IPTG
- CAS:367-93-1
- MF:C9H18O5S
- Structure:
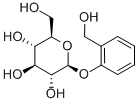
- Chemical Name:D-(-)-Salicin
- CAS:138-52-3
- MF:C13H18O7
- Structure:
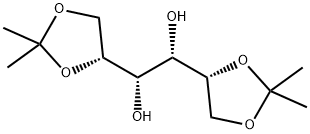
- Chemical Name:1,2:5,6-Bis-O-(1-methylethylidene)-D-mannitol
- CAS:1707-77-3
- MF:C12H22O6
- Structure:
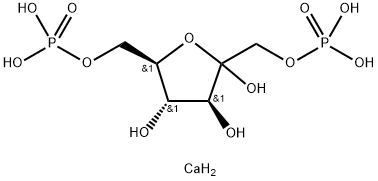
- Chemical Name:D-Fructose-1,6-diphoshate calcium salt
- CAS:103213-33-8
- MF:C6H16CaO12P2
- Structure:
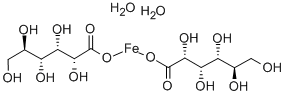
- Chemical Name:FERROUS GLUCONATE DIHYDRATE
- CAS:12389-15-0
- MF:C12H26FeO16
- Structure:
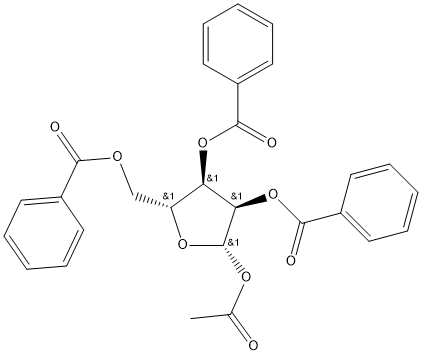
- Chemical Name:beta-D-Ribofuranose 1-acetate 2,3,5-tribenzoate
- CAS:6974-32-9
- MF:C28H24O9
- Structure:
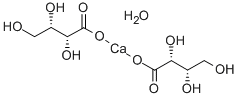
- Chemical Name:L-Threonic acid calcium salt
- CAS:70753-61-6
- MF:C8H14CaO10
- Structure:
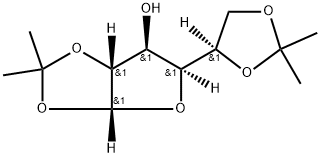
- Chemical Name:Diacetone-D-glucose
- CAS:582-52-5
- MF:C12H20O6
- Structure:
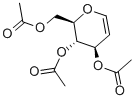
- Chemical Name:Tri-O-acetyl-D-glucal
- CAS:2873-29-2
- MF:C12H16O7
- Structure:
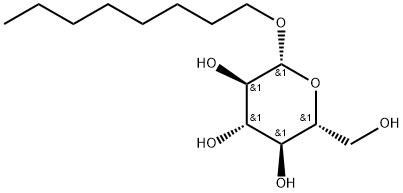
- Chemical Name:n-Octyl-β-D-glucopyranoside
- CAS:29836-26-8
- MF:C14H28O6
- Structure:
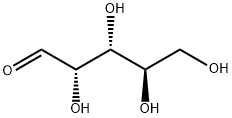
- Chemical Name:D-(-)-Arabinose
- CAS:10323-20-3
- MF:C5H10O5
- Structure:
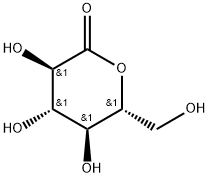
- Chemical Name:D-(+)-Glucono-1,5-lactone
- CAS:90-80-2
- MF:C6H10O6
- Structure:
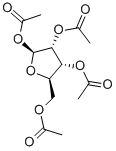
- Chemical Name:beta-D-Ribofuranose 1,2,3,5-tetraacetate
- CAS:13035-61-5
- MF:C13H18O9
- Structure:
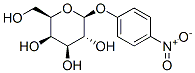
- Chemical Name:4-Nitrophenyl-beta-D-galactopyranoside
- CAS:3150-24-1
- MF:C12H15NO8
- Structure:
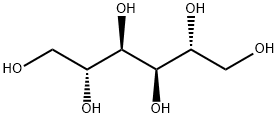
- Chemical Name:Mannitol
- CAS:87-78-5
- MF:C6H14O6
- Structure:
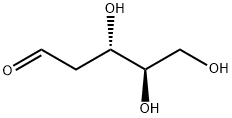
- Chemical Name:2-Deoxy-D-ribose
- CAS:533-67-5
- MF:C5H10O4
- Structure:
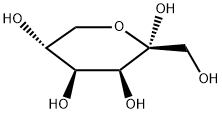
- Chemical Name:D-tagatose
- CAS:87-81-0
- MF:C6H12O6
- Structure:
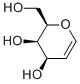
- Chemical Name:D-Galactal
- CAS:21193-75-9
- MF:C6H10O4
- Structure:
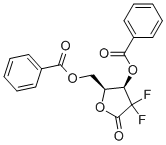
- Chemical Name:2-Deoxy-2,2-difluoro-D-erythro-pentafuranous-1-ulose-3,5-dibenzoate
- CAS:122111-01-7
- MF:C19H14F2O6
- Structure:
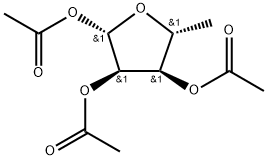
- Chemical Name:1,2,3-Triacetyl-5-deoxy-D-ribose
- CAS:62211-93-2
- MF:C11H16O7
- Structure:
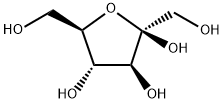
- Chemical Name:D(-)-Fructose
- CAS:57-48-7
- MF:C6H12O6
- Structure:
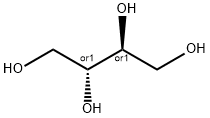
- Chemical Name:Erythritol
- CAS:149-32-6
- MF:C4H10O4
- Structure:
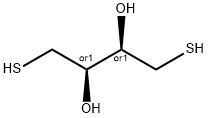
- Chemical Name:DL-Dithiothreitol
- CAS:3483-12-3
- MF:C4H10O2S2
- Structure:
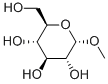
- Chemical Name:alpha-D-Methylglucoside
- CAS:97-30-3
- MF:C7H14O6
- Structure:
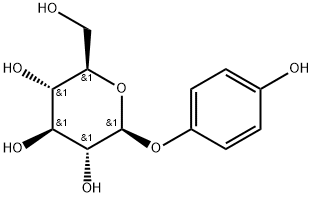
- Chemical Name:Arbutin
- CAS:497-76-7
- MF:C12H16O7
- Structure:
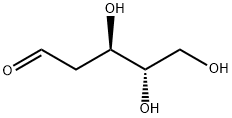
- Chemical Name:2-Deoxy-L-ribose
- CAS:18546-37-7
- MF:C5H10O4
- Structure:
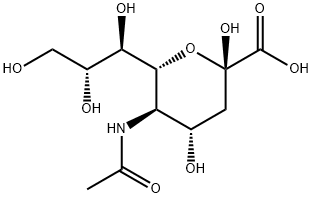
- Chemical Name:N-Acetylneuraminic acid
- CAS:131-48-6
- MF:C11H19NO9
- Structure:
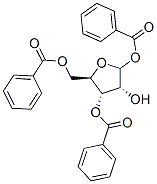
- Chemical Name:1,3,5-Tri-O-benzoyl-D-ribofuranose
- CAS:22224-41-5
- MF:C26H22O8
- Structure:
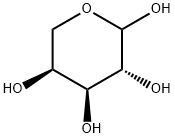
- Chemical Name:L-Arabinose
- CAS:87-72-9
- MF:C5H10O5
- Structure:
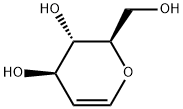
- Chemical Name:D-Glucal
- CAS:13265-84-4
- MF:C6H10O4
- Structure:
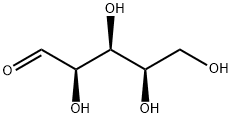
- Chemical Name:D(+)-Xylose
- CAS:58-86-6
- MF:C5H10O5
- Structure:
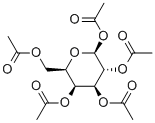
- Chemical Name:beta-D-Galactose pentaacetate
- CAS:4163-60-4
- MF:C16H22O11
- Structure:
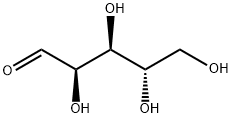
- Chemical Name:L-Arabinose
- CAS:5328-37-0
- MF:C5H10O5
- Structure:
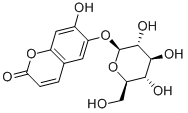
- Chemical Name:Esculin
- CAS:531-75-9
- MF:C15H16O9
- Structure:
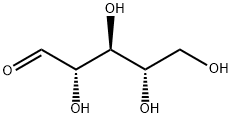
- Chemical Name:L-Ribose
- CAS:24259-59-4
- MF:C5H10O5
- Structure:
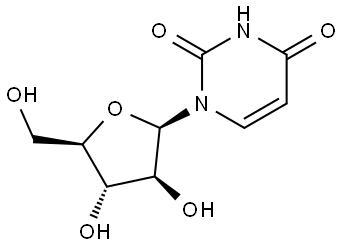
- Chemical Name:1-beta-D-Arabinofuranosyluracil
- CAS:3083-77-0
- MF:C9H12N2O6
- Structure:
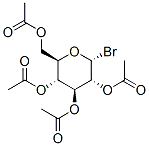
- Chemical Name:2,3,4,6-Tetra-O-acetyl-alpha-D-glucopyranosyl bromide
- CAS:572-09-8
- MF:C14H19BrO9
- Structure:
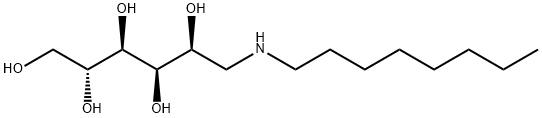
- Chemical Name:1-DEOXY-1-(OCTYLAMINO)-D-GLUCITOL
- CAS:23323-37-7
- MF:C14H31NO5
- Structure:
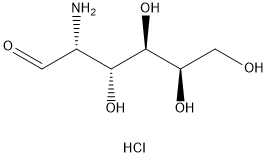
- Chemical Name:D(+)-Galactosamine hydrochloride
- CAS:1772-03-8
- MF:C6H14ClNO5
- Structure:
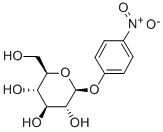
- Chemical Name:4-NITROPHENYL-BETA-D-GLUCOPYRANOSIDE
- CAS:2492-87-7
- MF:C12H15NO8
- Structure:
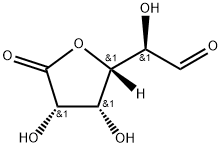
- Chemical Name:D-Glucurone
- CAS:32449-92-6
- MF:C6H8O6
- Structure:
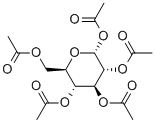
- Chemical Name:Glucose pentaacetate
- CAS:604-68-2
- MF:C16H22O11
- Structure:
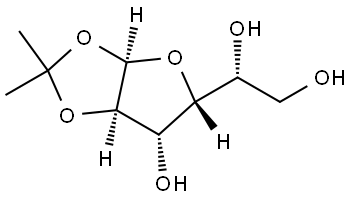
- Chemical Name:1,2-O-Isopropylidene-D-glucofuranose
- CAS:18549-40-1
- MF:C9H16O6
- Structure:
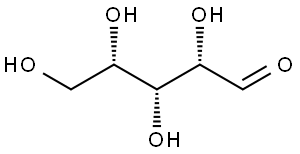
- Chemical Name:L-xylose
- CAS:609-06-3
- MF:C5H10O5
- Structure:
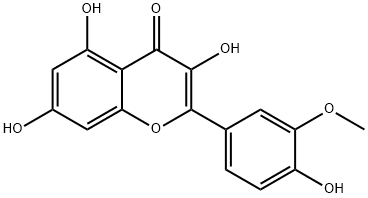
- Chemical Name:Isorhamnetin
- CAS:480-19-3
- MF:C16H12O7
- Structure:
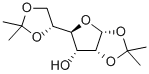
- Chemical Name:1,2:5,6-Di-O-isopropylidene-alpha-D-allofuranose
- CAS:2595-05-3
- MF:C12H20O6
- Structure:
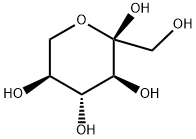
- Chemical Name:L-(-)-SORBOSE
- CAS:87-79-6
- MF:C6H12O6
- Structure:
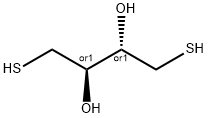
- Chemical Name:Dithioerythritol
- CAS:6892-68-8
- MF:C4H10O2S2
- Structure:
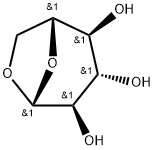
- Chemical Name:1,6-ANHYDRO-BETA-D-GLUCOPYRANOSE
- CAS:498-07-7
- MF:C6H10O5
- Structure:
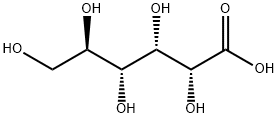
- Chemical Name:Gluconic acid
- CAS:526-95-4
- MF:C6H12O7
- Structure:
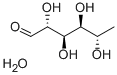
- Chemical Name:L(+)-Rhamnose monohydrate
- CAS:10030-85-0
- MF:C6H14O6
- Structure:
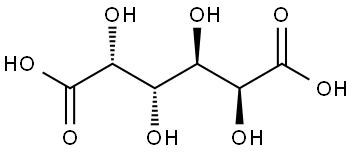
- Chemical Name:MUCIC ACID
- CAS:526-99-8
- MF:C6H10O8
- Structure:
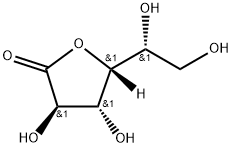
- Chemical Name:D-Gluconic acid, .gamma.-lactone
- CAS:1198-69-2
- MF:C6H10O6
- Structure:
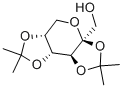
- Chemical Name:Diacetonefructose
- CAS:20880-92-6
- MF:C12H20O6
- Chemical Name:Arbutin
- CAS:
- MF:
- Structure:
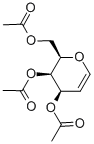
- Chemical Name:3,4,6-Tri-O-acetyl-D-galactal
- CAS:4098-06-0
- MF:C12H16O7
- Structure:
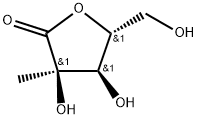
- Chemical Name:2-C-Methyl-D-ribono-1,4-lactone
- CAS:492-30-8
- MF:C6H10O5
- Structure:
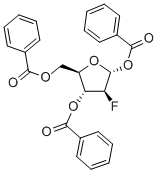
- Chemical Name:2-Deoxy-2-fluoro-1,3,5-tri-O-benzoyl-D-ribofuranose
- CAS:97614-43-2
- MF:C26H21FO7
- Structure:
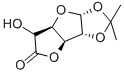
- Chemical Name:D-Glucurono-6,3-lactone acetonide
- CAS:20513-98-8
- MF:C9H12O6
- Structure:
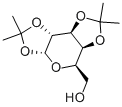
- Chemical Name:1,2:3,4-Di-O-isopropylidene-D-galactopyranose
- CAS:4064-06-6
- MF:C12H20O6
- Structure:
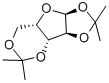
- Chemical Name:1,2:3,5-Di-O-isopropylidene-alpha-D-xylofuranose
- CAS:20881-04-3
- MF:C11H18O5
- Structure:
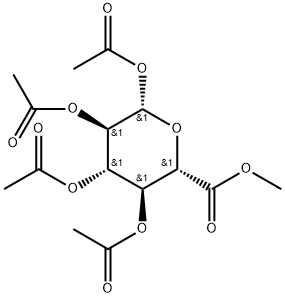
- Chemical Name:METHYL 1,2,3,4-TETRA-O-ACETYL-BETA-D-GLUCURONATE
- CAS:7355-18-2
- MF:C15H20O11
- Structure:
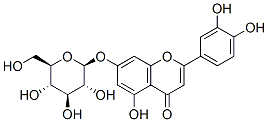
- Chemical Name:Cynaroside
- CAS:5373-11-5
- MF:C21H20O11
- Structure:
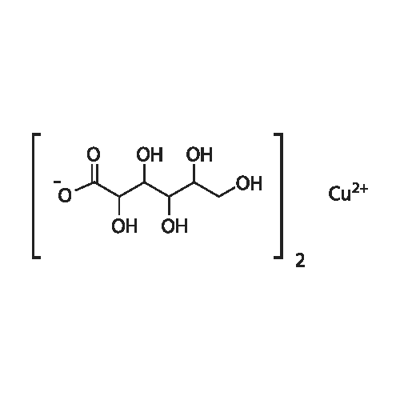
- Chemical Name:Copper(II) gluconate
- CAS:527-09-3
- MF:C12H22CuO14
- Structure:
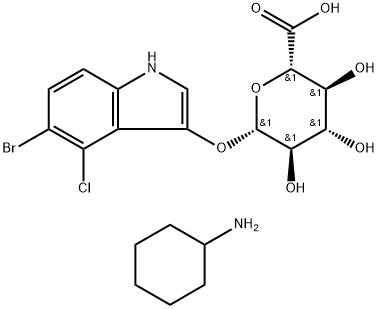
- Chemical Name:5-BROMO-4-CHLORO-3-INDOLYL B-D-
- CAS:114162-64-0
- MF:C20H26BrClN2O7
- Structure:
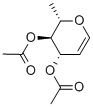
- Chemical Name:3,4-DI-O-ACETYL-6-DEOXY-L-GLUCAL
- CAS:34819-86-8
- MF:C10H14O5
- Structure:
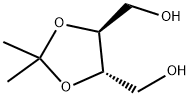
- Chemical Name:(+)-2,3-O-Isopropylidene-L-threitol
- CAS:50622-09-8
- MF:C7H14O4
- Structure:
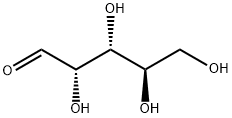
- Chemical Name:DL-Arabinose
- CAS:147-81-9
- MF:C5H10O5
- Structure:
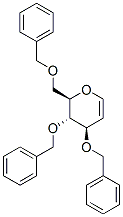
- Chemical Name:3,4,6-Tri-O-benzyl-D-glucal
- CAS:55628-54-1
- MF:C27H28O4
- Structure:
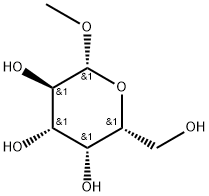
- Chemical Name:METHYL-BETA-D-GALACTOPYRANOSIDE
- CAS:1824-94-8
- MF:C7H14O6
- Structure:
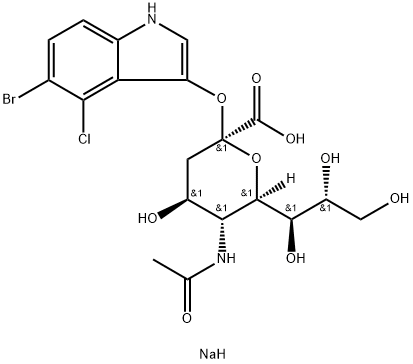
- Chemical Name:5-Bromo-4-chloro-3-indolyl-alpha-D-N-acetylneuraminic acid sodium salt
- CAS:160369-85-7
- MF:C19H23BrClN2NaO9
- Structure:
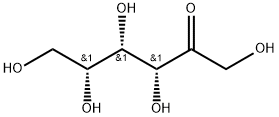
- Chemical Name:D-(+)-SORBOSE
- CAS:3615-56-3
- MF:C6H12O6
- Chemical Name:MANNAN
- CAS:51395-96-1
- MF:
- Structure:
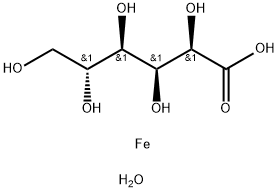
- Chemical Name:FERROUS GLUCONATE DIHYDRATE
- CAS:22830-45-1
- MF:C6H14FeO8
- Structure:
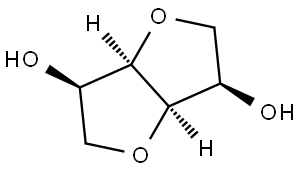
- Chemical Name:Isomannide
- CAS:641-74-7
- MF:C6H10O4
- Structure:
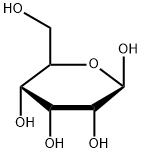
- Chemical Name:D-(+)-ALLOSE
- CAS:2595-97-3
- MF:C6H12O6
- Structure:
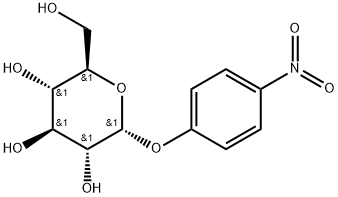
- Chemical Name:4-NITROPHENYL-ALPHA-D-GLUCOPYRANOSIDE
- CAS:3767-28-0
- MF:C12H15NO8
- Structure:
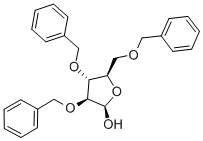
- Chemical Name:2,3,5-TRI-O-BENZYL-BETA-D-ARABINOFURANOSE
- CAS:60933-68-8
- MF:C26H28O5
- Structure:
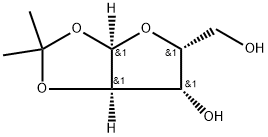
- Chemical Name:1,2-O-Isopropylidene-alpha-D-xylofuranose
- CAS:20031-21-4
- MF:C8H14O5
- Structure:
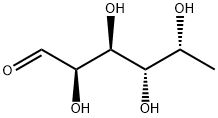
- Chemical Name:D-(+)-FUCOSE
- CAS:3615-37-0
- MF:C6H12O5
- Structure:
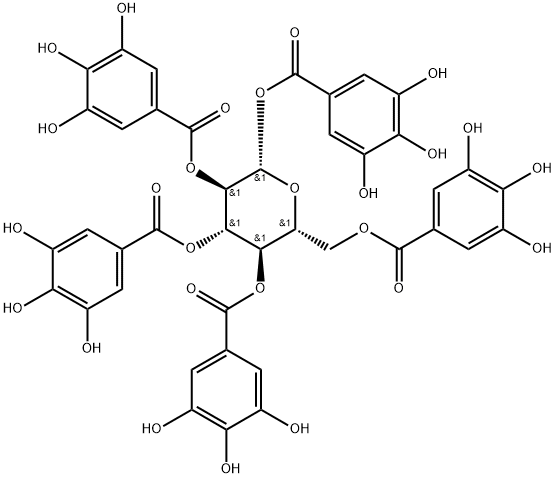
- Chemical Name:1,2,3,4,6-PENTA-O-GALLOYL-BETA-D-GLUCOPYRANOSE
- CAS:14937-32-7
- MF:C41H32O26
- Structure:
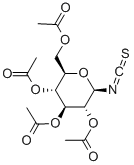
- Chemical Name:2,3,4,6-TETRA-O-ACETYL-BETA-D-GLUCOPYRANOSYL ISOTHIOCYANATE
- CAS:14152-97-7
- MF:C15H19NO9S
- Structure:
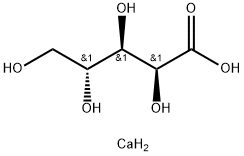
- Chemical Name:CALCIUM D-ARABONATE
- CAS:22373-09-7
- MF:C5H12CaO6
- Structure:
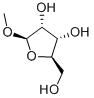
- Chemical Name:Methyl beta-D-ribofuranoside
- CAS:7473-45-2
- MF:C6H12O5
- Structure:
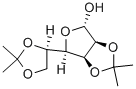
- Chemical Name:Diaceton-alpha-D-mannofuranose
- CAS:14131-84-1
- MF:C12H20O6
- Structure:
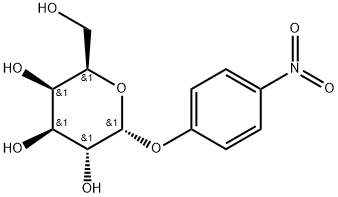
- Chemical Name:4-NITROPHENYL-ALPHA-D-GALACTOPYRANOSIDE
- CAS:7493-95-0
- MF:C12H15NO8
- Structure:
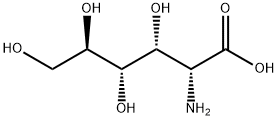
- Chemical Name:D-Glucosamic acid
- CAS:3646-68-2
- MF:C6H13NO6
- Structure:
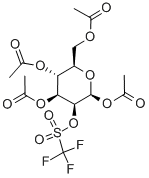
- Chemical Name:Mannose Triflate
- CAS:92051-23-5
- MF:C15H19F3O12S