Pyridine compound
Pyridine compound is one of the most widely developed and used heterocyclic compound. As an important fine chemical raw material, its derivatives mainly include alkyl pyridine, halogenated pyridine, aminopyridine, bromo pyridine, picoline , iodine pyridine, chloropyridine, nitropyridine-hydroxypyridine, benzyl pyridine, ethyl pyridine, pyridine cyano, fluoro pyridine, dihydropyridine, etc., in which the pesticide accounts for about 50% of the total consumption of pyridine products, feed additive about 30% and medicine and other areas 20%.
Pyridine is a colorless or slightly yellow liquid that has weak alkaline and special odor. It is an important chemical raw material and solvent, which naturally presents in coal tar, shale oil, gas and petroleum and is miscible with most of the water, alcohols, ethers, petroleum ether, oils, benzene, chloroform and other organic solvents.
The important derivatives of pyridine mainly include nicotinic acid, nicotinamide, isonicotinic hydrazide, nicotine, strychnine, and vitamin B6.
Pyridine has a close hexagonal structure that is similar to benzene. Because of the electron-withdrawing effect of the nitrogen atom in the ring, the electron density of 2,4,6 positions is less than that of 3,5 positions. In an acidic medium, electrophilic substitution reactions occur in the 3,5 positions and nucleophilic reactions, such as amination, alkylation, arylation, acylation, occur in the 2,4,6 positions. Pyridine is a weak tertiary amine, which can form different water-soluble salts with various acids (such as picric acid, perchloric acid or the like) in an ethanol solution. Due to the alkaline of pyridine, it can generate hydrochloride (C5H5N•HCl)with hydrochloric acid. In the effect of nickel catalyst at 200 ℃ and 15-30MPa, it can generate piperidine via hydrogenation or electrolysis reduction. The reduction of pyridine is easier than benzene, while the oxidation is more difficult than benzene. But we can oxidize pyridine by the catalysis of hydrogen peroxide or peroxy acids. N-pyridine oxide is an important pyridine derivative, which is easier for the happen of aryl group electrophilic substitution reaction because the oxidation of nitrogen atoms make the formation of positively charged pyridinium cation impossible. Electrophilic substitutions, such as pyridine nitration, sulfonation, halogenation, are relatively difficult, but the previous actions are slightly easier than halogenation. We can obtain 3,5-dichloro-pyridine or 3,4,5-trichloro pyridine via pyridine nitration and sulfonation at more than 200℃. Pyridine can form many crystalline complex compounds with various metal ions.
- Structure:
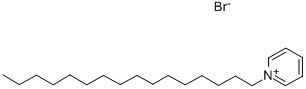
- Chemical Name:1-Hexadecylpyridinium bromide
- CAS:140-72-7
- MF:C21H38BrN
- Structure:
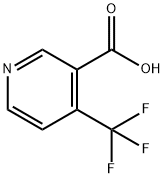
- Chemical Name:4-(Trifluoromethyl)nicotinic acid
- CAS:158063-66-2
- MF:C7H4F3NO2
- Structure:
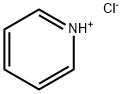
- Chemical Name:Pyridine hydrochloride
- CAS:628-13-7
- MF:C5H5N.ClH
- Structure:
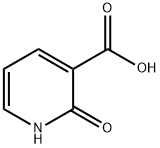
- Chemical Name:2-Hydroxynicotinic acid
- CAS:609-71-2
- MF:C6H5NO3
- Structure:
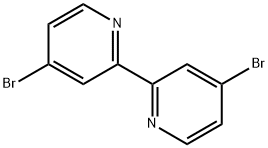
- Chemical Name:4,4'-DIBROMO-2,2'-BIPYRIDINE
- CAS:18511-71-2
- MF:C10H6Br2N2
- Structure:
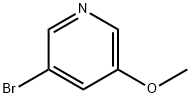
- Chemical Name:3-Bromo-5-methoxypyridine
- CAS:50720-12-2
- MF:C6H6BrNO
- Structure:
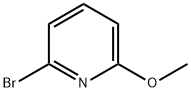
- Chemical Name:2-Bromo-6-methoxypyridine
- CAS:40473-07-2
- MF:C6H6BrNO
- Structure:
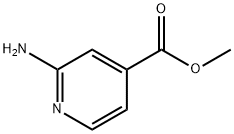
- Chemical Name:Methyl 2-aminopyridine-4-carboxylate
- CAS:6937-03-7
- MF:C7H8N2O2
- Structure:
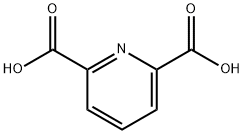
- Chemical Name:Pyridine-2,6-dicarboxylic acid
- CAS:499-83-2
- MF:C7H5NO4
- Structure:
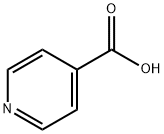
- Chemical Name:Isonicotinic acid
- CAS:55-22-1
- MF:C6H5NO2
- Structure:
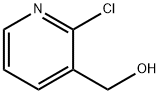
- Chemical Name:(2-Chloro-3-pyridinyl)methanol
- CAS:42330-59-6
- MF:C6H6ClNO
- Structure:
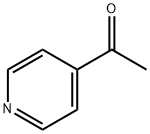
- Chemical Name:4-Acetylpyridine
- CAS:1122-54-9
- MF:C7H7NO
- Structure:
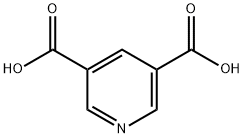
- Chemical Name:3,5-Pyridinedicarboxylic acid
- CAS:499-81-0
- MF:C7H5NO4
- Structure:
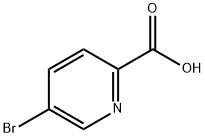
- Chemical Name:5-Bromo-2-pyridinecarboxylic Acid
- CAS:30766-11-1
- MF:C6H4BrNO2
- Structure:
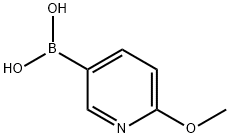
- Chemical Name:2-Methoxy-5-pyridineboronic acid
- CAS:163105-89-3
- MF:C6H8BNO3
- Structure:
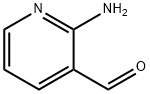
- Chemical Name:2-Amino-3-pyridinecarboxaldehyde
- CAS:7521-41-7
- MF:C6H6N2O
- Structure:
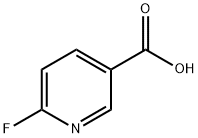
- Chemical Name:6-Fluoronicotinic acid
- CAS:403-45-2
- MF:C6H4FNO2
- Structure:
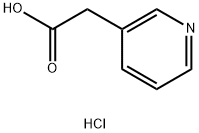
- Chemical Name:3-Pyridylacetic acid hydrochloride
- CAS:6419-36-9
- MF:C7H8ClNO2
- Structure:
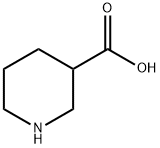
- Chemical Name:Nipecotic acid
- CAS:498-95-3
- MF:C6H11NO2
- Structure:
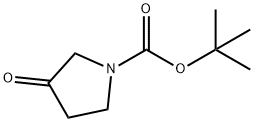
- Chemical Name:N-Boc-3-pyrrolidinone
- CAS:101385-93-7
- MF:C9H15NO3
- Structure:
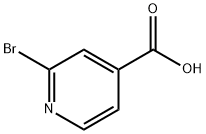
- Chemical Name:2-Bromopyridine-4-carboxylic acid
- CAS:66572-56-3
- MF:C6H4BrNO2
- Structure:
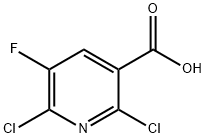
- Chemical Name:2,6-Dichloro-5-fluoronicotinic acid
- CAS:82671-06-5
- MF:C6H2Cl2FNO2
- Structure:
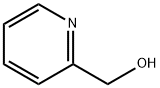
- Chemical Name:2-(Hydroxymethyl)pyridine
- CAS:586-98-1
- MF:C6H7NO
- Structure:
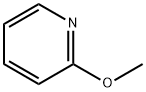
- Chemical Name:2-Methoxypyridine
- CAS:1628-89-3
- MF:C6H7NO
- Structure:
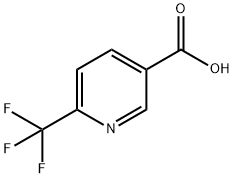
- Chemical Name:6-(Trifluoromethyl)nicotinic acid
- CAS:231291-22-8
- MF:C7H4F3NO2
- Structure:
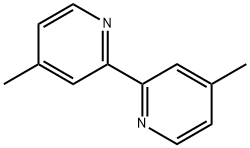
- Chemical Name:4,4'-Dimethyl-2,2'-bipyridyl
- CAS:1134-35-6
- MF:C12H12N2
- Structure:
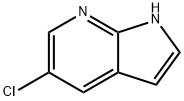
- Chemical Name:5-Chloro-7-azaindole
- CAS:866546-07-8
- MF:C7H5ClN2
- Structure:
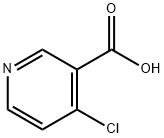
- Chemical Name:4-Chloronicotinic acid
- CAS:10177-29-4
- MF:C6H4ClNO2
- Structure:
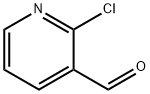
- Chemical Name:2-Chloro-3-pyridinecarboxaldehyde
- CAS:36404-88-3
- MF:C6H4ClNO
- Structure:
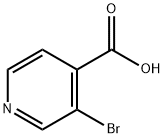
- Chemical Name:3-Bromoisonicotinic acid
- CAS:13959-02-9
- MF:C6H4BrNO2
- Structure:
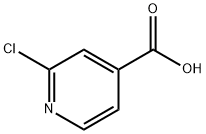
- Chemical Name:2-Chloro-4-pyridinecarboxylic acid
- CAS:6313-54-8
- MF:C6H4ClNO2
- Structure:
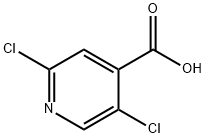
- Chemical Name:2,5-Dichloroisonicotinic acid
- CAS:88912-26-9
- MF:C6H3Cl2NO2
- Structure:
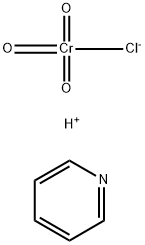
- Chemical Name:Pyridinium chlorochromate
- CAS:26299-14-9
- MF:C5H5N.ClCrO3.H
- Structure:
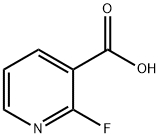
- Chemical Name:2-Fluoronicotinic acid
- CAS:393-55-5
- MF:C6H4FNO2
- Structure:
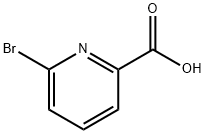
- Chemical Name:6-Bromopicolinic acid
- CAS:21190-87-4
- MF:C6H4BrNO2
- Structure:
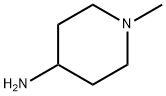
- Chemical Name:1-Methylpiperidin-4-amine
- CAS:41838-46-4
- MF:C6H14N2
- Structure:
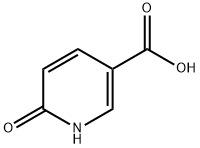
- Chemical Name:2-Hydroxy-5-pyridinecarboxylic acid
- CAS:5006-66-6
- MF:C6H5NO3
- Structure:
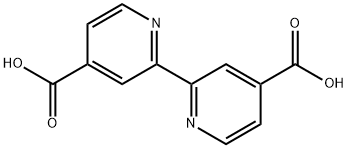
- Chemical Name:2,2'-Bipyridine-4,4'-dicarboxylic acid
- CAS:6813-38-3
- MF:C12H8N2O4
- Structure:
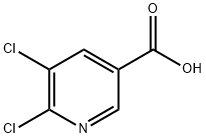
- Chemical Name:5,6-Dichloronicotinic acid
- CAS:41667-95-2
- MF:C6H3Cl2NO2
- Structure:
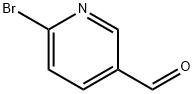
- Chemical Name:2-Bromopyridine-5-carbaldehyde
- CAS:149806-06-4
- MF:C6H4BrNO
- Structure:
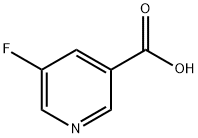
- Chemical Name:5-Fluoronicotinic acid
- CAS:402-66-4
- MF:C6H4FNO2
- Structure:
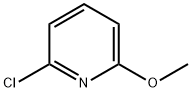
- Chemical Name:2-Chloro-6-methoxypyridine
- CAS:17228-64-7
- MF:C6H6ClNO
- Structure:
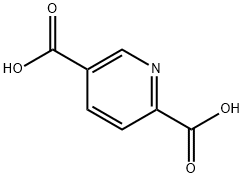
- Chemical Name:2,5-PYRIDINEDICARBOXYLIC ACID
- CAS:100-26-5
- MF:C7H5NO4
- Structure:
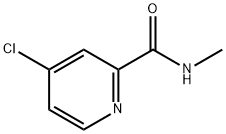
- Chemical Name:4-Chloro-N-methylpicolinamide
- CAS:220000-87-3
- MF:C7H7ClN2O
- Structure:
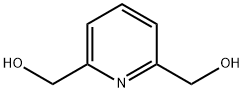
- Chemical Name:2,6-Pyridinedimethanol
- CAS:1195-59-1
- MF:C7H9NO2
- Structure:
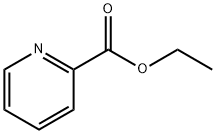
- Chemical Name:Ethyl picolinate
- CAS:2524-52-9
- MF:C8H9NO2
- Structure:
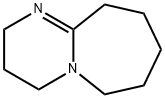
- Chemical Name:DBU
- CAS:6674-22-2
- MF:C9H16N2
- Structure:
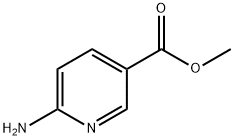
- Chemical Name:Methyl 6-aminonicotinate
- CAS:36052-24-1
- MF:C7H8N2O2
- Structure:
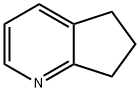
- Chemical Name:Cyclopenta[b]pyridine
- CAS:533-37-9
- MF:C8H9N
- Structure:
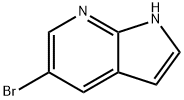
- Chemical Name:5-Bromo-7-azaindole
- CAS:183208-35-7
- MF:C7H5BrN2
- Structure:
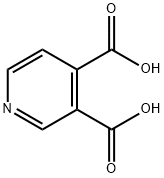
- Chemical Name:3,4-Pyridinedicarboxylic acid
- CAS:490-11-9
- MF:C7H5NO4
- Structure:
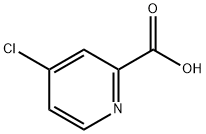
- Chemical Name:4-Chloropyridine-2-carboxylic acid
- CAS:5470-22-4
- MF:C6H4ClNO2
- Structure:
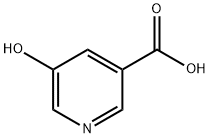
- Chemical Name:5-Hydroxynicotinic acid
- CAS:27828-71-3
- MF:C6H5NO3
- Structure:
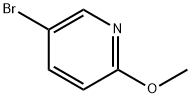
- Chemical Name:5-Bromo-2-methoxypyridine
- CAS:13472-85-0
- MF:C6H6BrNO
- Structure:
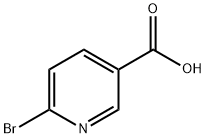
- Chemical Name:6-Bromonicotinic acid
- CAS:6311-35-9
- MF:C6H4BrNO2
- Structure:
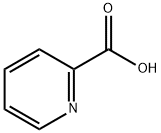
- Chemical Name:2-Picolinic acid
- CAS:98-98-6
- MF:C6H5NO2
- Structure:
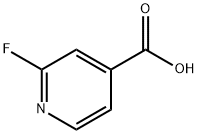
- Chemical Name:2-Fluoroisonicotinic acid
- CAS:402-65-3
- MF:C6H4FNO2
- Structure:
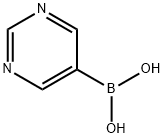
- Chemical Name:5-Pyrimidinylboronic acid
- CAS:109299-78-7
- MF:C4H5BN2O2
- Structure:
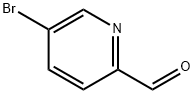
- Chemical Name:5-Bromopyridine-2-carbaldehyde
- CAS:31181-90-5
- MF:C6H4BrNO
- Structure:
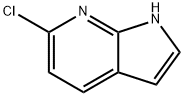
- Chemical Name:6-CHLORO-1H-PYRROLO[2,3-B]PYRIDINE
- CAS:55052-27-2
- MF:C7H5ClN2
- Structure:
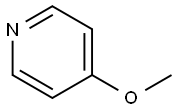
- Chemical Name:4-Methoxypyridine
- CAS:620-08-6
- MF:C6H7NO
- Structure:
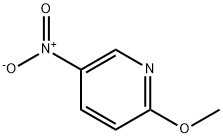
- Chemical Name:2-Methoxy-5-nitropyridine
- CAS:5446-92-4
- MF:C6H6N2O3
- Structure:
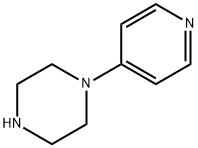
- Chemical Name:1-(4-Pyridyl)piperazine
- CAS:1008-91-9
- MF:C9H13N3
- Structure:
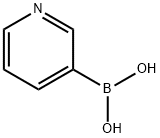
- Chemical Name:3-Pyridylboronic acid
- CAS:1692-25-7
- MF:C5H6BNO2
- Structure:
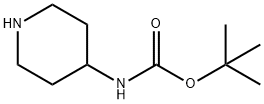
- Chemical Name:4-N-BOC-Aminopiperidine
- CAS:73874-95-0
- MF:C10H20N2O2
- Structure:
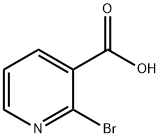
- Chemical Name:2-Bromonicotinic acid
- CAS:35905-85-2
- MF:C6H4BrNO2
- Structure:
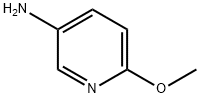
- Chemical Name:5-Amino-2-methoxypyridine
- CAS:6628-77-9
- MF:C6H8N2O
- Structure:
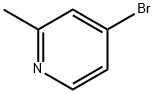
- Chemical Name:4-Bromo-2-methylpyridine
- CAS:22282-99-1
- MF:C6H6BrN
- Structure:
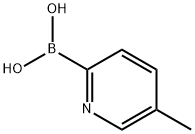
- Chemical Name:5-Methyl-2-pyridineboronic acid
- CAS:372963-49-0
- MF:C6H8BNO2
- Structure:
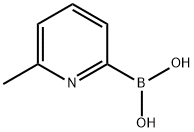
- Chemical Name:6-METHYLPYRIDINE-2-BORONIC ACID
- CAS:372963-50-3
- MF:C6H8BNO2
- Structure:
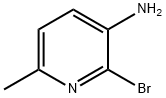
- Chemical Name:3-AMINO-2-BROMO-6-PICOLINE
- CAS:126325-53-9
- MF:C6H7BrN2
- Structure:
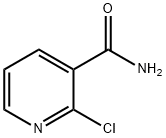
- Chemical Name:2-Chloronicotinamide
- CAS:10366-35-5
- MF:C6H5ClN2O
- Structure:
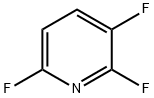
- Chemical Name:2,3,6-TRIFLUOROPYRIDINE
- CAS:3512-18-3
- MF:C5H2F3N
- Structure:
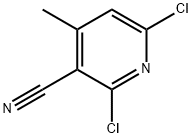
- Chemical Name:2,6-Dichloro-4-methylnicotinonitrile
- CAS:875-35-4
- MF:C7H4Cl2N2
- Structure:
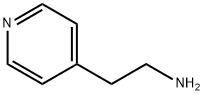
- Chemical Name:4-(2-Aminoethyl)pyridine
- CAS:13258-63-4
- MF:C7H10N2
- Structure:
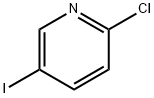
- Chemical Name:2-Chloro-5-iodopyridine
- CAS:69045-79-0
- MF:C5H3ClIN
- Structure:
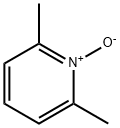
- Chemical Name:2,6-Dimethylpyridine N-oxide
- CAS:1073-23-0
- MF:C7H9NO
- Structure:
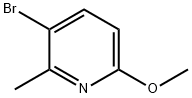
- Chemical Name:3-Bromo-6-methoxy-2-picoline
- CAS:126717-59-7
- MF:C7H8BrNO
- Structure:
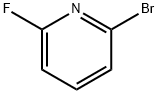
- Chemical Name:2-Bromo-6-fluoropyridine
- CAS:144100-07-2
- MF:C5H3BrFN
- Structure:
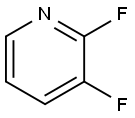
- Chemical Name:2,3-Difluoropyridine
- CAS:1513-66-2
- MF:C5H3F2N
- Structure:
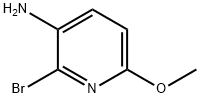
- Chemical Name:3-AMINO-2-BROMO-6-METHOXYPYRIDINE
- CAS:135795-46-9
- MF:C6H7BrN2O
- Structure:
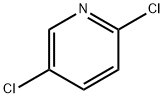
- Chemical Name:2,5-Dichloropyridine
- CAS:16110-09-1
- MF:C5H3Cl2N
- Structure:
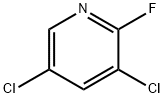
- Chemical Name:2-Fluoro-3,5-dichloropyridine
- CAS:823-56-3
- MF:C5H2Cl2FN
- Structure:
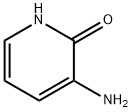
- Chemical Name:3-Amino-2(1H)-pyridinone
- CAS:33630-99-8
- MF:C5H6N2O
- Structure:
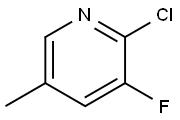
- Chemical Name:2-Chloro-3-fluoro-5-methylpyridine
- CAS:34552-15-3
- MF:C6H5ClFN
- Structure:
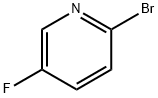
- Chemical Name:2-Bromo-5-fluoropyridine
- CAS:41404-58-4
- MF:C5H3BrFN
- Structure:
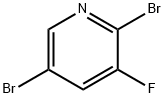
- Chemical Name:2,5-DIBROMO-3-FLUOROPYRIDINE
- CAS:156772-60-0
- MF:C5H2Br2FN
- Structure:
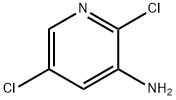
- Chemical Name:2,5-Dichloropyridin-3-amine
- CAS:78607-32-6
- MF:C5H4Cl2N2
- Structure:
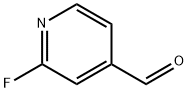
- Chemical Name:2-Fluoropyridine-4-carboxaldehyde
- CAS:131747-69-8
- MF:C6H4FNO
- Structure:
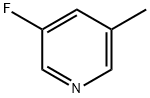
- Chemical Name:3-Fluoro-5-methylpyridine
- CAS:407-21-6
- MF:C6H6FN
- Structure:
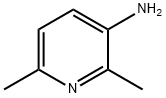
- Chemical Name:3-AMINO-2,6-DIMETHYLPYRIDINE
- CAS:3430-33-9
- MF:C7H10N2
- Structure:
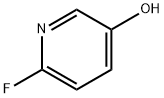
- Chemical Name:2-FLUORO-5-HYDROXYPYRIDINE
- CAS:55758-32-2
- MF:C5H4FNO
- Structure:
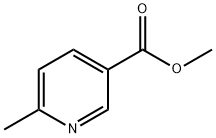
- Chemical Name:Methyl 6-methylnicotinate
- CAS:5470-70-2
- MF:C8H9NO2
- Structure:
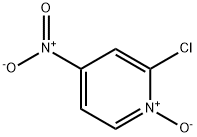
- Chemical Name:2-Chloro-4-nitropyridine 1-oxide
- CAS:14432-16-7
- MF:C5H3ClN2O3
- Structure:
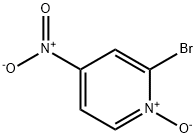
- Chemical Name:2-Bromo-4-nitropyridine 1-oxide
- CAS:52092-43-0
- MF:C5H3BrN2O3
- Structure:
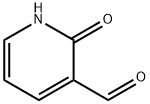
- Chemical Name:2-Hydroxynicotinaldehyde
- CAS:36404-89-4
- MF:C6H5NO2
- Structure:
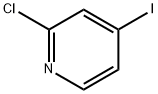
- Chemical Name:2-Chloro-4-iodopyridine
- CAS:153034-86-7
- MF:C5H3ClIN
- Structure:
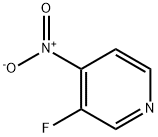
- Chemical Name:3-Fluoro-4-nitropyridine
- CAS:13505-01-6
- MF:C5H3FN2O2
- Structure:
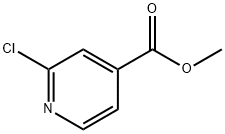
- Chemical Name:METHYL 2-CHLOROISONICOTINATE
- CAS:58481-11-1
- MF:C7H6ClNO2
- Structure:
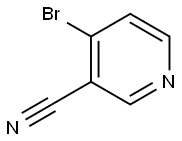
- Chemical Name:4-Bromo-3-cyanopyridine
- CAS:154237-70-4
- MF:C6H3BrN2