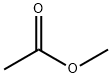
Methylacetat
Bezeichnung:Methylacetat
CAS-Nr79-20-9
Englisch Name:Methyl acetate
CBNumberCB9167443
SummenformelC3H6O2
Molgewicht74.08
MOL-Datei79-20-9.mol
Synonyma
Methylacetat
Essigsäuremethylester
Methylacetat physikalisch-chemischer Eigenschaften
| Schmelzpunkt | -98 °C (lit.) |
| Siedepunkt | 57-58 °C (lit.) |
| Dichte | 0.934 g/mL at 25 °C |
| Dampfdichte | 2.55 (vs air) |
| Dampfdruck | 165 mm Hg ( 20 °C) |
| Brechungsindex | n |
| FEMA | 2676 | METHYL ACETATE |
| Flammpunkt | 3.2 °F |
| storage temp. | no restrictions. |
| Löslichkeit | 250g/l |
| Aggregatzustand | Solution |
| Farbe | Clear colorless to slightly pale yellow |
| Relative polarity | 0.253 |
| Geruch (Odor) | Slightly acrid, sweet; fragrant. |
| PH | 7 |
| Biologische Quelle | synthetic |
| Geruchsart | ethereal |
| Odor Threshold | 1.7ppm |
| Kennzeichnung gefährlicher | F,Xi |
| R-Sätze: | 11-36-66-67 |
| S-Sätze: | 16-26-29-33 |
| RIDADR | UN 1231 3/PG 2 |
| OEB | A |
| OEL | TWA: 200 ppm (610 mg/m3), STEL: 250 ppm (760 mg/m3) |
| WGK Germany | 1 |
| RTECS-Nr. | AI9100000 |
| Selbstentzündungstemperatur | 936 °F |
| TSCA | Yes |
| HS Code | 2915 39 00 |
| HazardClass | 3 |
| PackingGroup | II |
| Giftige Stoffe Daten | 79-20-9(Hazardous Substances Data) |
| Toxizität | LD50 orally in Rabbit: > 5000 mg/kg LD50 dermal Rat > 2000 mg/kg |
| IDLA | 3,100 ppm [10% LEL] |


