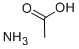
Ammoniumacetat
Bezeichnung:Ammoniumacetat
CAS-Nr631-61-8
Englisch Name:Ammonium acetate
CBNumberCB8438173
SummenformelC2H7NO2
Molgewicht77.08
MOL-Datei631-61-8.mol
Synonyma
Ammoniumacetat
Ammoniumacetat physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 110-112 °C (dec.) (lit.) |
| Siedepunkt | 138.46°C (rough estimate) |
| Dichte | 1.07 g/mL at 20 °C |
| chüttdichte | 410kg/m3 |
| Dampfdruck | 0.017-0.02Pa at 25℃ |
| Brechungsindex | 1.4350 (estimate) |
| Flammpunkt | 136 °C |
| storage temp. | Store at +15°C to +25°C. |
| Löslichkeit | H2O: 1 M at 20 °C, clear, colorless |
| Aggregatzustand | Solid |
| pka | 4.6(Acetic Acid), 9.3(Ammonium Hydroxide)(at 25℃) |
| Farbe | White |
| PH | 7(1 mM solution);7(10 mM solution);7(100 mM solution);6.95(1000 mM solution) |
| Säure-Base-Indikators(pH-Indikatoren) | 6.7 - 7.3 |
| Geruch (Odor) | Slight acetic acid odor |
| Wasserlöslichkeit | 1480 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| maximale Wellenlänge (λmax) | λ: 260 nm Amax: 0.015 λ: 280 nm Amax: 0.01 |
| S-Sätze: | 24/25 |
| RIDADR | UN 9079 |
| WGK Germany | 3 |
| RTECS-Nr. | AF3675000 |
| F | 3 |
| TSCA | Yes |
| HS Code | 29152900 |
| Giftige Stoffe Daten | 631-61-8(Hazardous Substances Data) |
| Toxizität | LD i.v. in mice: 1.8 mg (NH4+)/20g (Welch) |


