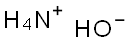
Ammoniak, wssrige Lsung
Bezeichnung:Ammoniak, wssrige Lsung
CAS-Nr1336-21-6
Englisch Name:Ammonium hydroxide
CBNumberCB1853050
SummenformelH5NO
Molgewicht35.05
MOL-Datei1336-21-6.mol
Synonyma
Ammoniak, wssrige Lsung
Ammoniakwasser
Ammoniumhydrat
Ammoniak, wssrige Lsung physikalisch-chemischer Eigenschaften
| Schmelzpunkt | -77°C |
| Siedepunkt | 36°C |
| Dichte | 0.91 g/mL at 20 °C |
| Dampfdichte | 1.2 (vs air) |
| Dampfdruck | 115 mmHg at 20 °C for 29% solution |
| storage temp. | 2-8°C |
| Löslichkeit | Water (Soluble) |
| Aggregatzustand | Liquid, Single Sub-Boiling Quartz Distillation |
| pka | 9.3(at 25℃) |
| Wichte | approximate 0.96 (10%, 15℃) |
| Farbe | Colorless |
| Geruch (Odor) | Strong pungent ammonia odor detectable at 17 ppm |
| PH | 10.09(1 mM solution);10.61(10 mM solution);11.12(100 mM solution); |
| Explosionsgrenze | 27% |
| Wasserlöslichkeit | Miscible with water. |
| maximale Wellenlänge (λmax) | λ: 260 nm Amax: 0.01 λ: 280 nm Amax: 0.01 |
| Merck | 14,494 |
| BRN | 3587154 |
| Kennzeichnung gefährlicher | C,N |
| R-Sätze: | 34-50-22 |
| S-Sätze: | 26-36/37/39-45-61 |
| RIDADR | UN 2672 8/PG 3 |
| WGK Germany | 2 |
| RTECS-Nr. | BQ9625000 |
| F | 34 |
| Selbstentzündungstemperatur | 690 °C (for ammonia) |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | III |
| HS Code | 28142000 |
| Giftige Stoffe Daten | 1336-21-6(Hazardous Substances Data) |
| Toxizität | LD50 oral (rat) 350 mg/kg PEL (OSHA) 35 ppm (27 mg/m3) TLV-TWA (ACGIH) 25 ppm (17 mg/m3) STEL (ACGIH) 35 ppm (27 mg/m3) |




