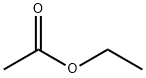
Ethylacetat
Bezeichnung:Ethylacetat
CAS-Nr141-78-6
Englisch Name:Ethyl acetate
CBNumberCB7255315
SummenformelC4H8O2
Molgewicht88.11
MOL-Datei141-78-6.mol
Synonyma
Ethylacetat
Essigs?ureethylester
Essigether
Ethylacetat physikalisch-chemischer Eigenschaften
| Schmelzpunkt | −84 °C(lit.) |
| Siedepunkt | 76.5-77.5 °C(lit.) |
| Dichte | 0.902 g/mL at 25 °C(lit.) |
| Dampfdichte | 3 (20 °C, vs air) |
| Dampfdruck | 73 mm Hg ( 20 °C) |
| Brechungsindex | n |
| FEMA | 2414 | ETHYL ACETATE |
| Flammpunkt | 26 °F |
| storage temp. | Store at +2°C to +25°C. |
| Löslichkeit | Miscible with ethanol, acetone, diethyl ether and benzene. |
| pka | 16-18(at 25℃) |
| Aggregatzustand | Liquid |
| Farbe | APHA: ≤10 |
| Wichte | 0.902 (20/20℃) |
| Geruch (Odor) | Pleasant fruity odor detectable at 7 to 50 ppm (mean = 18 ppm) |
| Relative polarity | 0.228 |
| Explosionsgrenze | 2.2-11.5%, 38°F |
| Geruchsart | ethereal |
| Biologische Quelle | synthetic |
| Odor Threshold | 0.87ppm |
| Wasserlöslichkeit | 80 g/L (20 ºC) |
| maximale Wellenlänge (λmax) | λ: 256 nm Amax: ≤1.00 λ: 275 nm Amax: ≤0.05 λ: 300 nm Amax: ≤0.03 λ: 325-400 nm Amax: ≤0.005 |
| JECFA Number | 27 |
| Merck | 14,3757 |
| BRN | 506104 |
| Henry's Law Constant | 0.39 at 5.00 °C, 0.58 at 10.00 °C, 0.85 at 15.00 °C, 1.17 at 20.00 °C, 1.58 at 25.00 °C (column stripping-UV, Kutsuna et al., 2005) |
| Expositionsgrenzwerte | TLV-TWA 400 ppm (~1400 mg/m3) (ACGIH, MSHA, and OSHA); IDLH 10,000 ppm (NIOSH). |
| Dielectric constant | 23.0(Ambient) |
| Stabilität | Stable. Incompatible with various plastics, strong oxidizing agents. Highly flammable. Vapour/air mixtures explosive. May be moisture sensitive. |
| InChIKey | XEKOWRVHYACXOJ-UHFFFAOYSA-N |
| LogP | 0.68-0.73 at 20-25℃ |
| Oberflächenspannung | 23.93mN/m at 293.15K |
| CAS Datenbank | 141-78-6(CAS DataBase Reference) |
| NIST chemische Informationen | Ethyl acetate(141-78-6) |
| EPA chemische Informationen | Ethyl acetate (141-78-6) |
| Kennzeichnung gefährlicher | F,Xi,Xn,T |
| R-Sätze: | 11-36-66-67-20/21/22-10-39/23/24/25-23/24/25-68/20/21/22 |
| S-Sätze: | 16-26-33-36/37-45-7-25 |
| RIDADR | UN 1173 3/PG 2 |
| OEB | A |
| OEL | TWA: 400 ppm (1400 mg/m3) |
| WGK Germany | 1 |
| RTECS-Nr. | AH5425000 |
| F | 1 |
| Selbstentzündungstemperatur | 427 °C |
| TSCA | Yes |
| HS Code | 2915 31 00 |
| HazardClass | 3 |
| PackingGroup | II |
| Giftige Stoffe Daten | 141-78-6(Hazardous Substances Data) |
| Toxizität | LD50 orally in rats: 11.3 ml/kg (Smyth) |
| IDLA | 2,000 ppm [10% LEL] |
Gefahreninformationscode (GHS)
-
Bildanzeige (GHS)


-
Alarmwort
Achtung
-
Gefahrenhinweise
H225:Flüssigkeit und Dampf leicht entzündbar.
H319:Verursacht schwere Augenreizung.
H336:Kann Schläfrigkeit und Benommenheit verursachen.
-
Sicherheit
P210:Von Hitze, heißen Oberflächen, Funken, offenen Flammen und anderen Zündquellenarten fernhalten. Nicht rauchen.
P233:Behälter dicht verschlossen halten.
P261:Einatmen von Staub vermeiden.
P280:Schutzhandschuhe/Schutzkleidung/Augenschutz tragen.
P303+P361+P353:BEI BERÜHRUNG MIT DER HAUT (oder dem Haar): Alle kontaminierten Kleidungsstücke sofort ausziehen. Haut mit Wasser abwaschen oder duschen.
P370+P378:Bei Brand: zum Löschen verwenden.
Ethyl acetate Chemische Eigenschaften,Einsatz,Produktion Methoden
-
ERSCHEINUNGSBILD
FARBLOSE FLüSSIGKEIT MIT CHARAKTERISTISCHEM GERUCH. -
PHYSIKALISCHE GEFAHREN
Die Dämpfe sind schwerer als Luft und können sich am Boden ausbreiten. Fernzündung möglich. -
CHEMISCHE GEFAHREN
Erhitzen kann zu sehr heftiger Verbrennung oder Explosion führen. Zersetzung unter Einfluss von UV-Licht, Säurenund Basen. Reagiert mit starken Oxidationsmitteln, Basen oder Säuren. Greift Aluminium und Kunststoffe an. -
ARBEITSPLATZGRENZWERTE
TLV: 400 ppm (als TWA); (ACGIH 2005).
MAK: 400 ppm, 1500 mg/m? Spitzenbegrenzung: überschreitungsfaktor I(2); Schwangerschaft: Gruppe C; (DFG 2005).
-
AUFNAHMEWEGE
Aufnahme in den Körper durch Inhalation der Dämpfe. -
INHALATIONSGEFAHREN
Beim Verdampfen bei 20°C kann schnell eine gesundheitsschädliche Kontamination der Luft eintreten. -
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION:
Die Substanz reizt die Augen und die Atemwege. Möglich sind Auswirkungen auf das Zentralnervensystem. Exposition weit oberhalb der Arbeitsplatzgrenzwerte kann zum Tod führen. -
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Die Flüssigkeit entfettet die Haut. -
LECKAGE
Gefahrenbereich verlassen! Ausgelaufene Flüssigkeit möglichst in abdichtbaren Behältern sammeln. Reste mit Sand oder inertem Absorptionsmittel aufnehmen und an einen sicheren Ort bringen. NICHT in die Kanalisation spülen. Persönliche Schutzausrüstung: Vollschutzanzug mit umgebungsluftunabhängigem Atemschutzgerät. -
R-Sätze Betriebsanweisung:
R11:Leichtentzündlich.
R36:Reizt die Augen.
R66:Wiederholter Kontakt kann zu spröder oder rissiger Haut führen.
R67:Dämpfe können Schläfrigkeit und Benommenheit verursachen.
R20/21/22:Gesundheitsschädlich beim Einatmen,Verschlucken und Berührung mit der Haut.
R10:Entzündlich.
R39/23/24/25:Giftig: ernste Gefahr irreversiblen Schadens durch Einatmen, Berührung mit der Haut und durch Verschlucken.
R23/24/25:Giftig beim Einatmen, Verschlucken und Berührung mit der Haut.
R68/20/21/22:Gesundheitsschädlich: Möglichkeit irreversiblen Schadens durch Einatmen,Berührung mit der Haut und durch Verschlucken. -
S-Sätze Betriebsanweisung:
S16:Von Zündquellen fernhalten - Nicht rauchen.
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S33:Maßnahmen gegen elektrostatische Aufladungen treffen.
S36/37:Bei der Arbeit geeignete Schutzhandschuhe und Schutzkleidung tragen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn möglich, dieses Etikett vorzeigen).
S7:Behälter dicht geschlossen halten.
S25:Berührung mit den Augen vermeiden. -
Aussehen Eigenschaften
C4H8O2; (Ethylacetat; Essigester; Essigether). Farblose, charakteristisch riechende Flüssigkeit. -
Gefahren für Mensch und Umwelt
Leichtentzündlich. Die Dämpfe sind viel schwerer als Luft und bilden mit Luft explosionsfähige Gemische. Mit Oxidationsmittel heftige Reaktion, ggf. Entzündung möglich.
Ethylacetat besitzt in niedrigen Dampfkonzentrationen einen angenehm fruchtargtigen Geruch, in mittleren Konzentrationen schleimhautreizende, in höheren Dosen narkotische Wirkung. Dasselbe tritt nach Verschlucken ein, wobei geringe Dosen krampflösend und auf die Atmung vertiefend wirken. Die Symptome nach Inhalation sind: Kratzen im Hals, Appetitlosigkeit, Magenschmerzen , Kopfschmerzen. Bei höheren Konzentrationen, je nach aufgenommener Menge, subnarkotische bis narkotische Symptome, evtl.. Atemlähmung. Lungenödeme sind möglich. Durch entfettende Wirkung sind Hautekzeme möglich.
Schwach wassergefährdender Stoff (WGK 1). -
Schutzmaßnahmen und Verhaltensregeln
Von Zündquellen fernhalten.
Nur in gut gelüfteten Bereichen verwenden.
Schutzhandschuhe nur als kurzzeitiger Spritzschutz. -
Verhalten im Gefahrfall
Mit saugfähigem, inertem Material z. Rench Rapid oder Chemozorb aufnehmen. Rückstände mit viel Wasser und Netzmittel wegspülen.
Wasser, Kohlendioxid, Trockenlöschmittel, Schaum. -
Erste Hilfe
Nach Augenkontakt: Bei Kontakt gründlich mit Wasser (mind. 10 Min.) spülen. Augenarzt konsultieren.
Nach Einatmen: Frischluft.
Nach Verschlucken: Sofort und wiederholt reichlich Wasser trinken. Erbrechen vermeiden (Aspirationsgefahr!). Arzt aufsuchen.
Nach Kleidungskontakt: Benetzte Kleidung sofort ausziehen.
Ersthelfer: siehe gesonderten Anschlag -
Sachgerechte Entsorgung
Als Sondermüll (halogenfreie Lösungsmittel) entsorgen. -
Beschreibung
Ethyl acetate (systematically, ethyl ethanoate, commonly abbreviated EtOAc or EA) is the organic compound with the formula CH3COOCH2CH3. This colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues, nail polish removers, decaffeinating tea and coffee, and cigarettes (see list of additives in cigarettes). Ethyl acetate is the ester of ethanol and acetic acid; it is manufactured on a large scale for use as a solvent. The combined annual production in 1985 of Japan, North America, and Europe was about 400,000 tons. In 2004, an estimated 1.3M tons were produced worldwide. -
Chemische Eigenschaften
Ethyl acetate has a pleasant ethereal fruity, brandy-like odor, reminiscent of pineapple, somewhat nauseating in high concentration. It has fruity sweet taste when freshly diluted in water. Ethyl acetate is probably one of the most used of all flavor chemicals by volume. Ethyl acetate is slowly decomposed by moisture and then acquires an acid status due to the acetic acid formed. -
Physikalische Eigenschaften
Clear, colorless, mobile liquid with a pleasant, sweet fruity odor. Experimentally determined detection and recognition odor threshold concentrations were 23 mg/m3 (6.4 ppmv) and 48 mg/m3 (13.3 ppmv), respectively (Hellman and Small, 1974). Cometto-Mu?iz and Cain (1991) reported an average nasal pungency threshold concentration of 67,300 ppmv. -
Occurrence
Although it has been reported present in some natural fruital aromas and in some distillates (rum, rum ether), it has not been reported yet as a constituent of essential oils; it has been identified also in the petals of Magnolia fuscata. Reported found in many foods including fresh and cooked apple, apricot, banana (169 ppm), sweet and sour cherry, citrus peel oils and juices, blueberry, cranberry, black currants, raspberry, blackberry, guava, passion fruit, melon, peaches, papaya, pineapple, cabbage, onion, leek, potato, tomato (3 to 6 ppm), clove, ginger, vinegar, breads, cheeses (0.2 to 0.8 ppm), butter (2 ppm), yogurt, milk, meats, cognac, beer (4 to 64 ppm), whiskies, cider, sherry, grape wines, rum, cocoa, coffee, tea, filberts, peanuts, popcorn, oats, honey, soybeans, coconut, olive oil (0.02 ppm) and olive. -
Verwenden
Ethyl acetate is used as a solvent for varnishes, lacquers, and nitrocellulose; as anartificial fruit flavor; in cleaning textiles;and in the manufacture of artificial silk andleather, perfumes, and photographic filmsand plates (Merck 1996). Ethyl Acetate is generally used as a solvent in organic reactions. Environmental contaminants; Food contaminants. -
Verwenden
Ethyl acetate is used primarily as a solvent and diluent, being favored because of its low cost, low toxicity, and agreeable odor. For example, it is commonly used to clean circuit boards and in some nail varnish removers (acetone and acetonitrile are also used). Coffee beans and tea leaves are decaffeinated with this solvent.It is also used in paints as an activator or hardener.[citation needed] Ethyl acetate is present in confectionery, perfumes, and fruits. In perfumes, it evaporates quickly, leaving only the scent of the perfume on the skin.
3 – 1 - Laboratory uses
In the laboratory, mixtures containing ethyl acetate are commonly used in column chromatography and extractions. Ethyl acetate is rarely selected as a reaction solvent because it is prone to hydrolysis and trans esterification.
3 – 2 - Occurrence in wines
Ethyl acetate is the most common ester in wine, being the product of the most common volatile organic acid — acetic acid, and the ethyl alcohol generated during the fermentation. The aroma of ethyl acetate is most vivid in younger wines and contributes towards the general perception of "fruitiness" in the wine.
3 – 3 - Entomological killing agent
In the field of entomology, ethyl acetate is an effective asphyxiant for use in insect collecting and study. In a killing jar charged with ethyl acetate, the vapors will kill the collected (usually adult) insect quickly without destroying it. Because it is not hygroscopic, ethyl acetate also keeps the insect soft enough to allow proper mounting suitable for a collection. -
Verwenden
Pharmaceutic aid (flavor); artificial fruit essences; solvent for nitrocellulose, varnishes, lacquers, and aeroplane dopes; manufacture of smokeless powder, artificial leather, photographic films and plates, artificial silk, perfumes; cleaning textiles, etc. -
Definition
ChEBI: Ethyl acetate is the acetate ester formed between acetic acid and ethanol. It has a role as a polar aprotic solvent, an EC 3.4.19.3 (pyroglutamyl-peptidase I) inhibitor, a metabolite and a Saccharomyces cerevisiae metabolite. It is an acetate ester, an ethyl ester and a volatile organic compound. -
Vorbereitung Methode
Ethyl acetate can be manufactured by the slow distillation of a mixture of ethanol and acetic acid in the presence of concentrated sulfuric acid. It has also been prepared from ethylene using an aluminum alkoxide catalyst. -
synthetische
Ethyl acetate is made by esterification of acetic acid with ethanol, from acetaldehyde, or by the direct addition of ethylene to acetic acid. BP started a 220,000 tonne/year plant in 2001 to operate the last of these processes, known as AVADA. Ethylene and acetic acid react in the presence of a heteropolyacid catalyst to give ethyl acetate at a claimed high selectivity and 99.97% purity. This is the world’s largest ethyl acetate plant and is motivated by its increasing use as a more “acceptable” solvent than hydrocarbons.
In some countries, where ethanol is expensive or there is surplus acetaldehyde capacity, ethyl acetate is made by a Tishchenko reaction. Sasol in South Africa was said to be investigating such a process in the early 2000s. Ethanol is a solvent for surface coatings, cleaning preparations, and cosmetics. Industrial ethanol is aerobically fermented to white vinegar (dilute acetic acid) of the type used for pickling. Gourmet vinegars—wine vinegar, cider vinegar, and so on, made by fermentation of alcoholic beverages—are also available. Ten percent of industrial ethanol production was used for vinegar in the United States in 2001. -
Vorbereitung Methode
Ethyl acetate is synthesized in industry mainly via the classic Fischer esterification reaction of ethanol and acetic acid. This mixture converts to the ester in about 65% yield at room temperature:
CH3CH2OH + CH3COOH ? CH3COOCH2CH3 + H2O
The reaction can be accelerated by acid catalysis and the equilibrium can be shifted to the right by removal of water. It is also prepared in industry using the Tishchenko reaction, by combining two equivalents of acetaldehyde in the presence of an alkoxide catalyst:
2 CH3CHO → CH3COOCH2CH3. -
Reaktionen
Ethyl acetate can be hydrolyzed in acidic or basic conditions to regain acetic acid and ethanol. The use of an acid catalyst accelerates the hydrolysis, which is subject to the Fischer equilibrium mentioned above. In the laboratory, and usually for illustrative purposes only, ethyl esters are typically hydrolyzed in a two step process starting with a stoichiometric amount of strong base, such as sodium hydroxide. This reaction gives ethanol and sodium acetate, which is unreactive toward ethanol:
CH3CO2C2H5 + Na OH → C2H5OH + CH3CO2Na
The rate constant is 0.111 dm3 / mol.sec at 25 °C. -
Aroma threshold values
Detection: 5 ppb to 5 ppm -
Allgemeine Beschreibung
Ethyl acetate, a carboxylate ester, is bio-friendly organic solvent with wide range of industrial applications. Its synthesis by reactive distillation and by acceptorless dehydrogenative dimerization of ethanol has been explored. Its utility as a less toxic alternative to diethyl ether in the formalin-ether (F-E) sedimentation procedure for intestinal parasites has been investigated. Its ability as an acyl acceptor in the immobilized lipase-mediated preparation of biodiesel from crude vegetable oils has been examined. The complete degradation of ethyl acetate to CO2 using manganese octahedral molecular sieve (OMS-2) has been investigated. -
Air & Water Reaktionen
Highly flammable. Slightly soluble in water. Ethyl acetate is slowly hydrolyzed by moisture. -
Reaktivität anzeigen
Ethyl acetate is also sensitive to heat. On prolonged storage, materials containing similar functional groups have formed explosive peroxides. Ethyl acetate may ignite or explode with lithium aluminum hydride. Ethyl acetate may also ignite with potassium tert-butoxide. Ethyl acetate is incompatible with nitrates, strong alkalis and strong acids. Ethyl acetate will attack some forms of plastics, rubber and coatings. Ethyl acetate is incompatible with oxidizers such as hydrogen peroxide, nitric acid, perchloric acid and chromium trioxide. Violent reactions occur with chlorosulfonic acid. . SOCl2 reacts with esters, such as Ethyl acetate, forming toxic SO2 gas and water soluble/toxic acyl chlorides, catalyzed by Fe or Zn (Spagnuolo, C.J. et al. 1992. Chemical and Engineering News 70(22):2.). -
Health Hazard
The acute toxicity of ethyl acetate is low. Ethyl acetate vapor causes eye, skin, and respiratory tract irritation at concentrations above 400 ppm. Exposure to high concentrations may lead to headache, nausea, blurred vision, central nervous system depression, dizziness, drowsiness, and fatigue. Ingestion of ethyl acetate may cause gastrointestinal irritation and, with larger amounts, central nervous system depression. Eye contact with the liquid can produce temporary irritation and lacrimation. Skin contact produces irritation. Ethyl acetate is regarded as a substance with good warning properties. No chronic systemic effects have been reported in humans, and ethyl acetate has not been shown to be a human carcinogen, reproductive, or developmental toxin -
Flammability and Explosibility
Ethyl acetate is a flammable liquid (NFPA rating = 3), and its vapor can travel a considerable distance to an ignition source and "flash back." Ethyl acetate vapor forms explosive mixtures with air at concentrations of 2 to 11.5% (by volume). Hazardous gases produced in ethyl acetate fires include carbon monoxide and carbon dioxide. Carbon dioxide or dry chemical extinguishers should be used for ethyl acetate fires. -
Chemische Reaktivität
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. -
Pharmazeutische Anwendungen
In pharmaceutical preparations, ethyl acetate is primarily used as a solvent, although it has also been used as a flavoring agent. As a solvent, it is included in topical solutions and gels, and in edible printing inks used for tablets.
Ethyl acetate has also been shown to increase the solubility of chlortalidone and to modify the polymorphic crystal forms obtained for piroxicam pivalate, mefenamic acid, and fluconazole,and has been used in the formulation of microspheres. Ethyl acetate has been used as a solvent in the preparation of a liposomal amphotericin B dry powder inhaler formulation.(9) Its use as a chemical enhancer for the transdermal iontophoresis of insulin has been investigated.
In food applications, ethyl acetate is mainly used as a flavoring agent. It is also used in artificial fruit essence and as an extraction solvent in food processing. -
Sicherheitsprofil
Potentially poisonous by ingestion. Toxicity depends upon alcohols in question, generally ethanol with methanol as a denaturant. A flammable liquid and dangerous fire hazard; can react vigorously with oxidzing materials. Moderate explosion hazard. See ETHANOL, METHYL ALCOHOL, and n-PROPYL ALCOHOL. -
Sicherheit(Safety)
Ethyl acetate is used in foods, and oral and topical pharmaceutical formulations. It is generally regarded as a relatively nontoxic and nonirritant material when used as an excipient.
However, ethyl acetate may be irritant to mucous membranes, and high concentrations may cause central nervous system depression. Potential symptoms of overexposure include irritation of the eyes, nose, and throat, narcosis, and dermatitis.
Ethyl acetate has not been shown to be a human carcinogen or a reproductive or developmental toxin.
The WHO has set an estimated acceptable daily intake of ethyl acetate at up to 25 mg/kg body-weight.
In the UK, it has been recommended that ethyl acetate be temporarily permitted for use as a solvent in food and that the maximum concentration consumed in food should be set at 1000 ppm.
LD50 (cat, SC): 3.00 g/kg
LD50 (guinea-pig, oral): 5.50 g/kg
LD50 (guinea-pig, SC): 3.00 g/kg
LD50 (mouse, IP): 0.709 g/kg
LD50 (mouse, oral): 4.10 g/kg
LD50 (rabbit, oral): 4.935 g/kg
LD50 (rat, oral): 5.62 g/kg -
Synthese
By reacting acetic acid and ethanol in the presence of sulfuric acid; by distillation of sodium potassium, or lead acetate with ethanol in the presence of sulfuric acid; by polymerizatin of acetaldehyde in the presence of aluminum ethylate or aluminum acetate as catalysts. -
mögliche Exposition
This material is used as a solvent for nitrocellulose and lacquer. It is also used in making dyes,flavoring and perfumery, and in smokeless powder manufacture -
Erste Hilfe
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately.If this chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPRif heart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, getmedical attention. Give large quantities of water andinduce vomiting. Do not make an unconscious personvomit -
Carcinogenicity
Ethyl acetate was not mutagenic in bacterial assays; it was not genotoxic in a number of in vivo assays but did cause chromosomal damage in hamster cells in vitro.
Ethyl acetate has a fruity odor detectable at 10ppm.
The 2003 ACGIH threshold limit valuetime- weighted average (TLV-TWA) for ethyl acetate is 400pm (1440mg/m3). -
Source
Identified among 139 volatile compounds identified in cantaloupe (Cucumis melo var. reticulates cv. Sol Real) using an automated rapid headspace solid phase microextraction method (Beaulieu and Grimm, 2001). -
Environmental Fate
Biological. Heukelekian and Rand (1955) reported a 5-d BOD value of 1.00 g/g which is 54.9% of the ThOD value of 1.82 g/g.
Photolytic. Reported rate constants for the reaction of ethyl acetate and OH radicals in the atmosphere (296 K) and aqueous solution are 1.51 x 10-12 and 6.60 x 10-13 cm3/molecule?sec, respectively (Wallington et al., 1988b).
Chemical/Physical. Hydrolyzes in water forming ethanol and acetic acid (Kollig, 1993). The estimated hydrolysis half-life at 25 °C and pH 7 is 2.0 yr (Mabey and Mill, 1978). -
Stoffwechsel
Ethyl acetate is hydrolysed to ethyl alcohol, which is then partly excreted in the expired air and urine. The rest is metabolized, the acetate fraction becoming incor porated in the body pool (Fassett, 1963). -
Lager
Ethyl acetate should be stored in an airtight container, protected from light and at a temperature not exceeding 30°C. Ethyl acetate is slowly decomposed by moisture and becomes acidic; the material can absorb up to 3.3% w/w water.
Ethyl acetate decomposes on heating to produce ethanol and acetic acid, and will emit acrid smoke and irritating fumes. It is flammable and its vapor may travel a considerable distance to an ignition source and cause a ‘flashback’.
The alkaline hydrolysis of ethyl acetate has been shown to be inhibited by polyethylene glycol and by mixed micelle systems. -
Versand/Shipping
UN1173 Ethyl acetate, Hazard Class: 3; Labels: 3-Flammable liquid. -
läuterung methode
The most common impurities in EtOAc are water, EtOH and acetic acid. These can be removed by washing with aqueous 5% Na2CO3, then with saturated aqueous CaCl2 or NaCl, and drying with K2CO3, CaSO4 or MgSO4. More efficient drying is achieved if the solvent is further dried with P2O5, CaH2 or molecular sieves before distillation. CaO has also been used. Alternatively, ethanol can be converted to ethyl acetate by refluxing with acetic anhydride (ca 1mL per 10mL of ester), the liquid is then fractionally distilled, dried with K2CO3 and redistilled. [Beilstein 2 III 127.] -
Toxicity evaluation
Ethyl acetate is rapidly hydrolyzed to ethanol and acetic acid. When ethyl acetate was injected intraperitoneal at 1.6 g kg-1, hydrolysis to acetic acid and ethanol occurred rapidly. The biological half-life value of the conversion of ethyl acetate to ethanol was found to be between 5 and 10 min. At doses higher than 1.6 g kg-1 in rats the rate of hydrolysis exceeded the ethanol oxidation leading to the ethanol accumulation in the vascular system. -
Inkompatibilitäten
Ethyl acetate can react vigorously with strong oxidizers, strong alkalis, strong acids, and nitrates to cause fires or explosions. It also reacts vigorously with chlorosulfonic acid, lithium aluminum hydride, 2-chloromethylfuran, and potassium tert-butoxide. -
Filter für giftige stoffe
The initial threshold screening level (ITSL) for ethyl acetate is 3200 μg/m3, with annual averaging time. -
Waste disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≧100 kg/ mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. -
Regulatory Status
Included in the FDA Inactive Ingredients Database (oral tablets and sustained-action tablets; topical and transdermal preparations). Included in nonparenteral medicines licensed in the UK (tablets, topical solutions, and gels). Ethyl acetate is also accepted for use in food applications in a number of countries including the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Ethyl acetate Upstream-Materialien And Downstream Produkte
Upstream-Materialien
1of2
Downstream Produkte
- self curing adhesive SL-B404
- D-Glucosepentakis[3,4-dihydroxy-5-[(trihydroxy-3,4,5-benzoyl)oxy]benzoat]
- (Ethyl-3-oxobutyrato-O1',O3)bis(propan-2-olato)aluminium
- adhesive JX-18-1
- adhesive M-861 for polyolefin plastics
- Hexadecyltrimethylammoniumchlorid
- ETHYL PICOLINOYLACETATE
- Ethyl-3-(4-pyridyl)-3-oxopropionat
- Enoximone
- N-Acetylethylenediamine
- Water-proof adhesive
- Triphenylsilanol
- special adhesive JA-501 for laminating packaging materials
- O-Benzyl-N-[(tert-butoxy)carbonyl]-L-tyrosin
- 2-(4-Ethoxyphenyl)-2-methylpropanol
- 2-Amino-6-bromopyridine
- Tea polyphenol
- 3-METHYL-4-PYRIDINECARBOXYLIC ACID
- N-ETHYL 3-NITROBENZENESULFONAMIDE
- special adhesive JA-502 for aluminum-plastics laminating tape
- wealant XY-2
- 1-(Thiazol-2-yl)ethan-1-on
- Methyl 4-bromo-3-nitrobenzoate
- 4-FLUOROBENZYL ISOCYANATE
- 4(1H)-Pyrimidinone, 2-methyl- (8CI,9CI)
- 2-(2-FORMYL-PHENOXY)-PROPIONIC ACID
- adhesive No.1 for shrink packaging
- Piperidin-3-ol
- Natrium-[2S-[2α,5α,6β(S*)]]-6-(aminophenylacetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptan-2-carboxylat
- N-(tert-Butoxycarbonyl)glycin
1of8
Ethylacetat Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global(540)Suppliers
Region
-
Henan Xiangduo Industry Co., Ltd.
Telefon +86-15981848961<br/>+86-15981848961
E-Mail sales@xiangduochem.com
-
Shandong Yanshuo Chemical Co., Ltd.
Telefon +86-18678179670<br/>+86-18615116763
E-Mail sales@yanshuochem.com
-
Telefon +86-85511178;<br/>+86-85511178;
E-Mail peter68@ptchemgroup.com
-
Telefon +86-18994963526<br/>+8618994963526
E-Mail 1346073549@qq.com
-
Telefon
E-Mail tp@aladdinsci.com
-
Telefon
E-Mail tp@aladdinsci.com
-
Runte International Trade Limited
Telefon 19565631292<br/>19565631292
E-Mail lucky@sdruntechem.com
-
Hebei Chuanghai Biotechnology Co., Ltd
Telefon +86-15531157085<br/>+86-15531157085
E-Mail abby@chuanghaibio.com
-
Hebei Chuanghai Biotechnology Co,.LTD
Telefon +86-13131129325
E-Mail sales1@chuanghaibio.com
-
Hebei Dangtong Import and export Co LTD
Telefon +86-86-4001020630<br/>+8619831957301
E-Mail admin@hbdangtong.com
1of2
