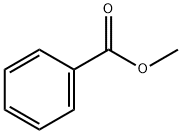
Methylbenzoat
Bezeichnung:Methylbenzoat
CAS-Nr93-58-3
Englisch Name:Methyl benzoate
CBNumberCB8252119
SummenformelC8H8O2
Molgewicht136.15
MOL-Datei93-58-3.mol
Synonyma
Methylbenzoat
Benzoes?uremethylester
Niobe?l
Methylbenzoat physikalisch-chemischer Eigenschaften
| Schmelzpunkt | -12 °C (lit.) |
| Siedepunkt | 198-199 °C (lit.) |
| Dichte | 1.088 g/mL at 20 °C (lit.) |
| Dampfdichte | 4.68 (vs air) |
| Dampfdruck | <1 mm Hg ( 20 °C) |
| Brechungsindex | n |
| FEMA | 2683 | METHYL BENZOATE |
| Flammpunkt | 181 °F |
| storage temp. | Store at +5°C to +30°C. |
| Löslichkeit | ethanol: soluble60%, clear (1mL/4ml) |
| Aggregatzustand | Liquid |
| Farbe | Clear colorless to pale yellow |
| Wichte | 1.087~1.095 (20℃) |
| Geruch (Odor) | at 1.00 % in dipropylene glycol. phenolic wintergreen almond floral cananga |
| Geruchsart | phenolic |
| Explosionsgrenze | 8.6-20%(V) |
| Wasserlöslichkeit | <0.1 g/100 mL at 22.5 ºC |
| Merck | 14,6024 |
| Kennzeichnung gefährlicher | Xn |
| R-Sätze: | 22 |
| S-Sätze: | 36 |
| RIDADR | UN 2938 |
| WGK Germany | 1 |
| RTECS-Nr. | DH3850000 |
| Selbstentzündungstemperatur | 510 °C |
| TSCA | Yes |
| HS Code | 29163100 |
| Giftige Stoffe Daten | 93-58-3(Hazardous Substances Data) |
| Toxizität | LD50 orally in rats: 3.43 g/kg (Smyth) |

