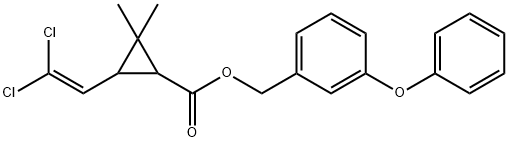
Перметрин
- английское имяPermethrin
- CAS №52645-53-1
- CBNumberCB8312237
- ФормулаC21H20Cl2O3
- мольный вес391.29
- EINECS258-067-9
- номер MDLMFCD00041809
- файл Mol52645-53-1.mol
| Температура плавления | 34-35°C |
| Температура кипения | bp0.05 220° |
| плотность | 1.19 |
| давление пара | 7×10-5 Pa (20 °C) |
| показатель преломления | 1.6500 (estimate) |
| Fp | 10 °C |
| температура хранения | 0-6°C |
| растворимость | DMF: 33 mg/ml; DMSO: 16 mg/ml; Ethanol: 14 mg/ml |
| форма | Liquid |
| цвет | Light yellow to yellow |
| Растворимость в воде | insoluble |
| Мерк | 13,7257 |
| БРН | 5765325 |
| Стабильность | Stable. Incompatible with strong oxidising agents. |
| ИнЧИКей | RLLPVAHGXHCWKJ-UHFFFAOYSA-N |
| LogP | 6.500 |
| Справочник по базе данных CAS | 52645-53-1(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 4-5 |
| FDA UNII | 509F88P9SZ |
| Код УВД | P03AC04,P03AC54 |
| МАИР | 3 (Vol. 53) 1991 |
| Справочник по химии NIST | Permethrin(52645-53-1) |
| Пестициды Закон о свободе информации (FOIA) | Permethrin |
| Система регистрации веществ EPA | Permethrin (52645-53-1) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn,N,T,F | |||||||||
| Заявления о рисках | 20/22-43-50/53-39/23/24/25-23/24/25-11-51/53-36-20/21/22 | |||||||||
| Заявления о безопасности | 13-24-36/37/39-60-61-45-36/37-16-7-26 | |||||||||
| РИДАДР | UN1230 3/PG 2 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | GZ1255000 | |||||||||
| кода HS | 29162090 | |||||||||
| Банк данных об опасных веществах | 52645-53-1(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in female rats: 3801 mg/kg (Metker); LD50 in 8 day old rats, male adult rats (mg/kg): 340.5, 1500.0 orally (Cantalamessa) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
H302+H332:Вредно при проглатывании или при вдыхании.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
Перметрин химические свойства, назначение, производство
Описание
Yellow-brown to brown liquid, which sometimes tends to crystallize at room temperature (technical grade).
Permethrin, a pyrethroid insecticide with little mammalian toxicity, has been marketed as a tick repellent. Permethrin is the only synthetic pyrethroid that is used worldwide for head lice. The spray formulation is the most widely available form. Once applied to clothing, it is stable through several wash cycles. It is also suitable for treating sleeping bags, tents, and fabric used for insect screening. A liquid concentrate has been used to impregnate fabric for as long as the life of the garment. Permethrin is particularly helpful in the prevention of tick and chigger bites. These crawling arthropods must travel across the treated fabric, whereas flying insects tend to be attracted directly to exposed skin. The combination of a DEET-containing repellent and permethrin-treated clothing is generally highly ffective against a wide range of biting arthropods.
Химические свойства
Crystalline SolidИспользование
Labelled Permethrin (P288500). Synthetic pyrethroid insectide, more stable to light and at least as active as the natural pyrethrins and with low mammalian toxicity.Показания
Permethrin is a synthetic pyrethroid active against lice, ticks, mites, and fleas. It acts on neural cellmembranes, delaying repolarization and causing paralysis. The compound has no reported adverse properties, is heat and light stable, has 70% to 80% ovicidal activity, and leaves an active residue on the scalp.Permethrin 0.5% spray kills ticks and repels mosquitoes and stable flies. It is broken down when applied to skin and, hence, should be applied to clothing, shoes, tents, and so on. Spray only enough to moisten the material. Spray on clothing at least 2 hours before wearing. One treatment should last through a few washings.
Определение
ChEBI: A cyclopropanecarboxylate ester in which the esterifying alcohol is 3-phenoxybenzyl alcohol and the cyclopropane ring is substituted with a 2,2-dichlorovinyl group and with gem-dimethyl groups.Общее описание
Pale brown liquid. Relatively water insoluble. Used as an insecticide.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
A pyrethroid derivative.Контактные аллергены
Pyrethroids, also called pyrethrinoids, are neurotoxic synthetic compounds used as insecticides, with irritant properties. Cypermethrin and fenvalerate have been reported as causing positive allergic patch tests, but only fenvalerate was relevant in an agricultural worker.Фармаколо?гия
Permethrin is a synthetic pyrethroid that interferes with the influx of sodium through cell membranes, leading to neurologic paralysis and death of the mite. There is minimal percutaneous absorption (<2%), which is rapidly hydrolyzed and excreted in the urine. Permethrin 5% dermal cream (Elimite) is applied for 8 to 12 hours to the entire body from the chin down and is then washed off.Клиническое использование
Permethrin is 3-(2,2-Dichloroethenyl)-2,2-dimethylcyclopropanecarboxylicacid (3-phenoxyphenyl)methylester or 3-(phenoxyphenyl)methyl (±)-cis, trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate(Nix). This synthetic pyrethrinoid compound is more stablechemically than most natural pyrethrins and is at least as activeas an insecticide. Of the four isomers present, the1(R),trans and 1(R),cis-isomers are primarily responsiblefor the insecticidal activity. The commercial product is amixture consisting of 60% trans and 40% cis racemic isomers.It occurs as colorless to pale yellow low-melting crystalsor as a pale yellow liquid and is insoluble in water butsoluble in most organic solvents.Permethrin exerts a lethal action against lice, ticks, mites,and fleas. It acts on the nerve cell membranes of the parasitesto disrupt sodium channel conductance. It is used as apediculicide for the treatment of head lice. A single applicationof a 1% solution effects cures in more than 99% ofcases. The most frequent side effect is pruritus, which occurredin about 6% of the patients tested.
Профиль безопасности
Poison by inhalation, intravenous, and intracerebral routes. Moderately toxic by ingestion. Experimental reproductive effects. Mutation data reported. A skin irritant. When heated to decomposition it emits toxic fumes of Cl-. See also ESTERSЭкологическая судьба
Soil. Permethrin biodegraded rapidly via hydrolysis yielding 3-(2,2-dichloroethenyl)- 2,2-dimethylcyclopropanecarboxylic acid and 3-phenoxybenzyl alcohol (Kaufman et al., 1981). The reported half-life in soil containing 1.3–51.3% organic matter and pH 4.2–7.7 is <38 days (Worthing and Hance, 1991)In lake water, permethrin degraded more rapidly than in flooded sediment to transand cis-(dichlorovinyl)dimethylcyclopropanecarboxylic acid. The cis isomer was more stable toward biological and chemical degradation than the trans isomer (Sharom and Solom
Plant. Metabolites identified in cotton leaves included trans-hydroxypermethrin, 2′- hydroxypermethrin, 4′-hydroxypermethrin, dichlorovinyl acid, dichlorovinyl acid conjugates, hydroxydichlorovinyl acid, hydroxydichlorovinyl acid conjugates, phe
Dislodgable residues of permethrin on cotton leaves 0, 24, 48, 72 and 96 hours after application (1.1 kg/ha) were (cis:trans): 0.26:0.38, 0.24:0.34, 0.22:0.32, 0.16:0.24 and 0.15:0.21 μg/m2, respectively (Buck et al., 1980).
Photolytic. Photolysis of permethrin in aqueous solutions containing various solvents (acetone, hexane and methanol) under UV light (λ >290 nm) or on soil in sunlight initially resulted in the isomerization of the cyclopropane moiety and ester cl
Метаболический путь
Permethrin was one of the first photostable pyrethroids suitable for field use.It served as a precursor for others such as its α-cyano analogue, cypermethrin. As it lacks the cyano group it has no chirality at the acarbon atom and therefore consists of a mixture of only four isomers. It is hydrolysed more readily than cypermethrin because it is an ester of a primary alcohol.Much of the early research on the metabolism of the synthetic, photostable pyrethroids was conducted by Casida and co-workers on permethrin. The published results greatly assisted subsequent work on the analogues. This is stressed because some of the information reported below will draw on the metabolic schemes given in the cypermethrin entry.
The environmental fate of permethrin and its metabolism in plants, insects, animals and fish have been reported. The insecticide is metabolised by ester cleavage to its constituent acid and alcohol and subsequent conjugation of these. The primary metabolites are also subject to oxidation prior to conjugation. Permethrin itself is also subject to oxidation, the cisisomers more so than the trans-isomers because the former is more slowly hy droly sed.
Перметрин запасные части и сырье
сырьё
- Циклопропан
- Трихлорацетальдегид
- ethyl 3-(2,2-dichlorovinyl)-2,2-dimethyl-1-cyclopropanecarboxylate
- Hydrogen chloride ethanol solution
- P-толуолсульфоновая кислота
1of5
запасной предмет
Перметрин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +8617732866630 | China | 18147 | 58 | |
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | |
| +8618092446649 | China | 1143 | 58 | |
| +86-0571-58605786 +8617758008775 |
China | 47 | 58 | |
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 | |
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 008657128800458; +8615858145714 |
China | 7738 | 55 | |
| 025-85710122 17714198479 | CHINA | 885 | 55 |