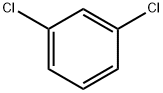
1,3-дихлорбензол
- английское имя1,3-Dichlorobenzene
- CAS №541-73-1
- CBNumberCB8853781
- ФормулаC6H4Cl2
- мольный вес147
- EINECS208-792-1
- номер MDLMFCD00000573
- файл Mol541-73-1.mol
химическое свойство
| Температура плавления | -25--22 °C (lit.) |
| Температура кипения | 172-173 °C (lit.) |
| плотность | 1.288 g/mL at 25 °C (lit.) |
| давление пара | 5 mm Hg ( 38.8 °C) |
| показатель преломления | n |
| Fp | 146 °F |
| температура хранения | 2-8°C |
| растворимость | Difficult to mix. |
| форма | Liquid |
| цвет | Clear colorless to slightly yellow |
| Запах | Pleasant odor |
| Растворимость в воде | 0.0123 g/100 mL (25 ºC) |
| Точка замерзания | -24℃ |
| Мерк | 14,3055 |
| БРН | 956618 |
| констант закона Генри | 2.14 at 20.00 °C (inert gas stripping, Hovorka and Dohnal, 1997) |
| Диэлектрическая постоянная | 5.0 |
| Стабильность | Stability Combustible. Incompatible with strong oxidizing agents, aluminium, aluminium alloys. Moisture-sensitive. |
| ИнЧИКей | ZPQOPVIELGIULI-UHFFFAOYSA-N |
| LogP | 3.53 at 22℃ |
| Справочник по базе данных CAS | 541-73-1(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 2 |
| FDA UNII | 75W0WNE5FP |
| МАИР | 3 (Vol. 73) 1999 |
| Справочник по химии NIST | Benzene, 1,3-dichloro-(541-73-1) |
| Система регистрации веществ EPA | m-Dichlorobenzene (541-73-1) |
| UNSPSC Code | 41116105 |
| NACRES | NA.22 |
больше
| Коды опасности | Xn,N,T,F | |||||||||
| Заявления о рисках | 22-51/53-39/23/24/25-23/24/25-11 | |||||||||
| Заявления о безопасности | 61-45-36/37-16-7 | |||||||||
| РИДАДР | UN 3082 9/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | CZ4499000 | |||||||||
| Температура самовоспламенения | >500 °C | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1(b) | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29036990 | |||||||||
| Банк данных об опасных веществах | 541-73-1(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 580 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
H411:Токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P273:Избегать попадания в окружающую среду.
P301+P312+P330:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии. Прополоскать рот.
1,3-дихлорбензол химические свойства, назначение, производство
Физические свойства
1,3-Dichlorobenzene is a clear, colorless liquid with a disinfectant or musty-type odor. Soluble in alcohol, ether, insoluble in water. At 40 °C, the average odor threshold concentration and the lowest concentration at which an odor was detected were 170 and 177 μg/L, respectively. At 25 °C, the lowest concentration at which a taste was detected was 190 μg/L, respectively (Young et al., 1996).Использование
1,3-Dichlorobenzene is used in organic synthesis that can synthesize fungicides imazalil, propiconazole, epiconazole, and insecticides insectaphos. It is also used in the synthesis of 1,2-diaminobenzene, in addition, it is also used as a solvent in the fields of dyes, medicine, resin, rubber and so on.Использование
1,3-Dichlorobenzene is used in medicine, and dyes. And it is also used to study the effect of solvent vapor pressure on the vertical nanowire of C60 molecules1. 1,3-Dichlorobenzene, a chlorinated aromatic hydrocarbon, can be detected in environmental samples using an advanced Fourier transform infrared spectroscopy-attenuated total reflectance (FTIR-ATR) sensor.Определение
ChEBI: 1,3-dichlorobenzene is a dichlorobenzene carrying chloro substituents at positions 1 and 3. Used as a fumigant, insecticide, and chemical intermediate.Подготовка
1,3-Dichlorobenzene is prepared from o-chloroaniline through diazotization and displacement reaction.Add o-chloroaniline and hydrochloric acid into the reaction pot, mix evenly below 25°C, cool to 0°C, drop in sodium nitrite solution, control the temperature at 0-5°C, stop adding when the potassium iodide starch indicator turns blue, and obtain diazonium salt solution. Then add the diazonium salt solution into the hydrochloric acid solution of cuprous chloride at 0~5℃, stir well, heat up to 60~70℃ and react for 1h, after cooling, let stand for stratification, and use 5% sodium hydroxide for the oil layer. Repeated washing with water, dehydration with anhydrous calcium chloride, fractional distillation, and collection of fractions at 177-183 °C to obtain the product 1,3-dichlorobenzene.
Общее описание
Colorless liquid. Sinks in water.Реакции воздуха и воды
1,3-Dichlorobenzene is sensitive to moisture. Insoluble in water.Профиль реактивности
1,3-Dichlorobenzene is incompatible with oxidizing agents and aluminum and its alloys. Above the flash point, explosive vapor-air mixtures may be formed.Угроза здоровью
INHALATION: Causes headache, drousiness, unsteadiness. Irritating to mucous membranes. EYES: Severe irritation. SKIN: Severe irritation. INGESTION: Irritation of gastric mucosa, nausea, vomiting, diarrhea, abdominal cramps and cyanosis.Пожароопасность
Special Hazards of Combustion Products: Irritating vapors including hydrogen chloride are produced.Профиль безопасности
Moderately toxic by intraperitoneal route. Mutation data reported. When heated to decomposition it emits toxic fumes of Cl-. See also oDICHLOROBENZENE and p DICHLOROBENZENE.Возможный контакт
The major uses of o-DCB are as a process solvent in the manufacturing of toluene diisocyanate and as an intermediate in the synthesis of dyestuffs, herbicides, and degreasers. p-Dichlorbenzene is used primarily as a moth repellant, a mildew control agent; space deodorant; and in insecticides, which accounts for 90% of the total production of this isomer. Information is not available concerning the production and use of m-DCB. However, it may occur as a contaminant of o-or p-DCB formulations. Both o-and p-isomers are produced almost entirely as by-products during the production of monochlorobenzeneЭкологическая судьба
Biological. When 1,3-dichlorobenzene was statically incubated in the dark at 25 °C with yeast extract and settled domestic wastewater inoculum, significant biodegradation with gradual acclimation was followed by a deadaptive process in subsequent subcultures. At a concentration of 5 mg/L, 59, 69, 39, and 35% losses were observed after 7, 14, 21, and 28-d incubation periods, respectively. At a concentration of 10 mg/L, percent losses were virtually unchanged. After 7, 14, 21, and 28-d incubation periods, percent losses were 58, 67, 31, and 33, respectively (Tabak et al., 1981).Photolytic. The sunlight irradiation of 1,3-dichlorobenzene (20 g) in a 100-mL borosilicate glass-stoppered Erlenmeyer flask for 56 d yielded 520 ppm trichlorobiphenyl (Uyeta et al., 1976).
Chemical/Physical. Anticipated products from the reaction of 1,3-dichlorobenzene with atmospheric ozone or OH radicals are chlorinated phenols, ring cleavage products, and nitro compounds (Cupitt, 1980). Based on an assumed base-mediated 1% disappearance after 16 d at 85 oC and pH 9.70 (pH 11.26 at 25 oC), the hydrolysis half-life was estimated to be >900 yr (Ellington et al., 1988). 1,3-Dichlorobenzene (0.17–0.23 mM) reacted with OH radicals in water (pH 8.7) at a rate of 5.0 x 109/M·sec (Haag and Yao, 1992).
Перевозки
m-DCB: UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. United States DOT Regulated Marine Pollutant. UN3077 Environmentally hazardous substances, solis, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical NameRequired. UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name RequiredМетоды очистки
Wash it with aqueous 10% NaOH, then with water until neutral, dry and distil it. Conductivity material (ca 10-10 mhos) has been prepared by refluxing over P2O5 for 8hours, then fractionally distilling, and storing with activated alumina. m-Dichlorobenzene dissolves rubber stoppers. [Beilstein 5 IV 657.]Несовместимости
For o-DCB and m-DCB: acid fumes, chlorides, strong oxidizers; hot aluminum, or aluminum alloys. For p-DCB: Strong oxidizers; although, incompatibilities for this chemical may also include other materials listed for o-DCB.Утилизация отходов
Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal1,3-дихлорбензол запасные части и сырье
сырьё
1of4
запасной предмет
1of3
1,3-дихлорбензол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-57-80696996 +86-19816093969 |
China | 16 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-371-66670886 | China | 19902 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +undefined-21-51877795 | China | 32965 | 60 | ||
| +86-75521030354 +86-18688942810 |
China | 802 | 55 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | ||
| 13867897135 | CHINA | 923 | 58 |
1,3-дихлорбензол Обзор)
- 2,5-дихлорфенола
- 2,3-дихлорбензойной кислоты
- 2,6-дихлорбензонитрила
- 2,4-дихлорбензил хлорид
- 1-ХЛОРО-3-НИТРОБЕНЗОЛ
- 2,4-дихлорфеноксиуксусная кислота
- 2,6-дихлорфенол
- 1,4-Дихлорбензол
- 2,3-дихлорфенол
- 1,3-дибромбензола
1of4