Simplicef (veterinary drug)
Vantin (human drug)
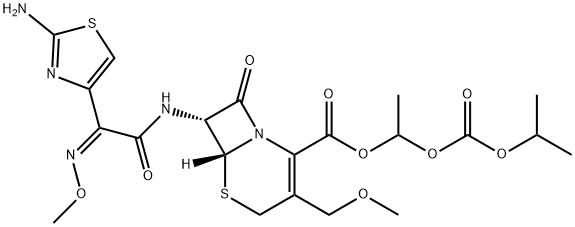
Cefpodoxime proxetil is an orally active, broad-spectrum cephalosporin especially useful
in the treatment of bacterial infections in children. It is a prodrug converted by esterases
in the GI walls to cefpodoxime, which reportedly has a bacteriostatic spectrum
comparable to injectable third-generation cephem antibiotics.
A metabolite of Cefpodoxime Proxetil. An antibacterial.
An antibacterial. A broad spectrum, orally absorbed third generation cephalosporin, ester prodrug of the active free acid metabolite, Cefpodoxime
A broad spectrum antibiotic. Cefpodoxime proxetil is commonly used in clinical in vitro microbiological antimicrobial susceptibility tests (panels, discs, and MIC strips) against gram positive and gram negative microbial isolates. Medical microbiologists use AST results to recommend antibiotic treatment options for infected patients. Product is to be used for research and development only.
ChEBI: The 1-[(isopropoxycarbonyl)oxy]ethyl (proxetil) ester prodrug of cefpodoxime. After swallowing, hydrolysis of the ester group occurs in the intestinal epithelium, to release active cefpodoxime in the bloodstream. It is used to treat acute otitis media, pha
yngitis, and sinusitis.
A suspension of 30 g of 7-amino-3-methoxymethyl-3-cephem-4-carboxylic
acid in 300 ml acetone is mixed with 18.6 g 1,8-diazabicyclo[5.4.0]undec-7-
ene. The solution obtained mixed at ca. 0°C with 261 g of a 14% toluene
solution of 1-iodoethylisopropyl carbonate. After 4 hours the solution is poured
onto a mixture of 600 ml of water and 21 ml conc. HCl. The pH of mixture is
adjusted to ca. 1.0. The aqueous phase is extracted with 200 ml of hexane,
mixed with 700 ml ethyl acetate and pH is adjusted to ca. 8.2. A solution of
7-amino-3-methoxymethyl-3-cephem-4-carboxylic acid 1-
(isopropoxycarbonyloxy)ethyl ester is obtained. Diastereoisomeric ratio
B/(A+B)=0.49 (B is more apolar of the two diastereoisomers).
To a solution of 37.4 g of 7-amino-3-methoxymethyl-3-cephem-4-carboxylic
acid 1-(isopropoxycarbonyloxy)ethyl ester in 689 ml ethyl acetate at 2-3°C is
added for 25 min 0.105 moles Z-(2-formamidothiazol-4-yl)-methoxy-acetyl
chloride hydrochloride. After 25 min pH is adjusted to ca. 6.5-7.3. After 1
hour the organic layer is washed with water and concentrated. It was obtained
a crude 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 7-(((2Z)-(2-
amino-4-thiazolyl)(methoxyimino)acetyl)amino)-3-(methoxymethyl)-8-oxo-,
1-(((1-methylethoxy)carbonyl)oxy)ethyl ester, (6R,7R)- (Cefpodoxime
proxetil). Diastereoisomeric ratio 0.49.
For purification 5 g of cefpodoxime proxetil are added to a mixture of 35 ml of
methanol and 0.6 ml conc. H2SO4. The mixture is stirred for 90 min and
slowly added during 25 min to a mixture of 2.1 g sodium bicarbonate and 400
ml water. The suspension obtained is stirred for 1 hour and the precipitate is
isolated through a suction filter, washed with water and dried over phosphorus
pentoxide at 35°C in a vacuum. 5-Thia-1-azabicyclo(4.2.0)oct-2-ene-2-
carboxylic acid, 7-(((2Z)-(2-amino-4-thiazolyl)(methoxyimino)acetyl)amino)-
3-(methoxymethyl)-8-oxo-, 1-(((1-methylethoxy)carbonyl)oxy)ethyl ester,
(6R,7R)-; (Cefpodoxime proxetil) is obtained in a diastereoisomeric ratio
0.528.
Vantin (Pharmacia & Upjohn);Banan.
Cefpodoxime proxetil (Vantin) is the isopropyloxycarbonylethylester of the third-generation cephalosporincefpodoxime. This orally active prodrug derivative is hydrolyzedby esterases in the intestinal wall and in theplasma to provide cefpodoxime. Tablets and a powder forthe preparation of an aqueous suspension for oral pediatricadministration are available. The oral bioavailability of cefpodoximefrom the proxetil is estimated to be about 50%.Administration of the prodrug with food enhances its absorption.The plasma half-life is 2.2 hours, which permitsadministration on a twice-daily schedule.
Cefpodoxime is a broad-spectrum cephalosporin withuseful activity against a relatively wide range of Grampositiveand Gram-negative bacteria. It is also resistant tomany β-lactamases. Its spectrum of activity includes S.pneumoniae, Streptococcus pyogenes, S. aureus, H. influenzae,M. catarrhalis, and Neisseria spp. Cefpodoxime is alsoactive against members of the Enterobacteriaceae family,including E. coli, K. pneumoniae, and P. mirabilis. It thusfinds use in the treatment of upper and lower respiratory infections,such as pharyngitis, bronchitis, otitis media, andcommunity-acquired pneumonia. It is also useful for thetreatment of uncomplicated gonorrhea.
Common side effects of Cefpodoxime proxetil include: headache, nausea, vomiting, diarrhoea, stomach pain, insomnia, vaginal swelling, redness, irritation, burning or itching. Severe may include: rash, itching, measles, difficulty breathing or swallowing, fever, sore throat, chills, or other signs of an infection coming back; swollen ankles or feet, dark urine, yellowing of the skin, easy bruising or bleeding, kidney problems. Seek medical attention if you have a serious adverse reaction.
Veterinary Drugs and Treatments
In dogs, cefpodoxime is indicated for the treatment of skin infections
caused by Staphylococcus intermedius, Staphylococcus aureus,
Streptococcus canis, E. coli, Proteus mirabilis, and Pasteurella multocida.
Although not currently approved for cats, it may also be useful
as well.