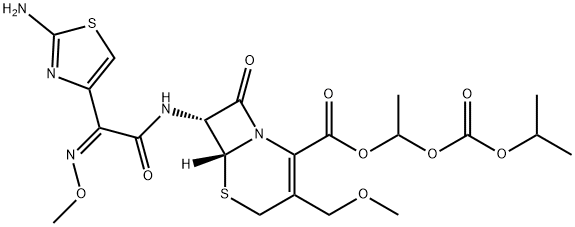
Antibiotics Cefpodoxime proxetil: Uses, Pharmacokinetics and Side effects
Uses of Cefpodoxime proxetil
Cefpodoxime proxetil is an oral, third-generation cephalosporin antibiotic drug. It works by stopping the growth of bacteria. It is used to treat a wide range of bacterial infections such as bronchitis (infection of the airway tubes leading to the lungs); pneumonia; gonorrhoea (a sexually transmitted disease); and infections of the skin, ears, sinuses, throat, tonsils and urinary tract. In addition, Cefpodoxime proxetil is generally well tolerated in paediatric patients and has shown good clinical efficacy in paediatric patients with a variety of infectious diseases, including acute otitis media, tonsillitis and/or pharyngitis. However, it is not indicated for viral infections (e.g., common cold, influenza).
Mechanism of action of Cefpodoxime proxetil
Cefpodoxime proxetil is converted to cefpodoxime, its active form, in the body. Like other cephalosporins, cefpodoxime stops bacteria from multiplying by preventing bacteria from forming the walls that surround them. The walls are necessary to protect bacteria from their environment and to keep the contents of the bacterial cell together; most bacteria cannot survive without a cell wall. Cefpodoxime is active against a wide spectrum of bacteria such as Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes (the cause of strep throat), Streptococcus agalactiae, Hemophilus influenzae, Moraxella catarrhalis, E. coli, Klebsiella, Proteus mirabilis, Proteus vulgaris, Providencia rettgeri, Haemophilus parainfluenzae, and Neisseria gonorrhoeae.
Pharmacokinetics of Cefpodoxime proxetil
Cefpodoxime proxetil is an orally absorbed broad spectrum third generation cephalosporin antibacterial. It is a prodrug that is de-esterified in vivo to its active metabolite, cefpodoxime. After single- and multiple-dose (12-hourly) administration of cefpodoxime proxetil in the therapeutic dose range of 100 to 400mg of cefpodoxime equivalents, average peak plasma concentrations of cefpodoxime range from 1.0 to 4.5 mg/L and occur between 1.9 and 3.1 hours after administration. The half-life of cefpodoxime ranges from 1.9 to 2.8 hours. The absolute bio-availability of cefpodoxime proxetil tablets is 50%, and absorption is enhanced by concomitant administration of food. Raising gastric pH by pretreatment with antacids or H2-receptor antagonists results in reduced absorption. Binding of cefpodoxime to human plasma or serum protein is low (18 to 23%), suggesting that cefpodoxime should readily transfer across the capillary lining into tissues. Cefpodoxime undergoes minimal metabolism in humans. Drug not absorbed is degraded in the gastrointestinal tract and excreted in the faeces. As expected for a drug eliminated primarily by renal excretion, the disposition of cefpodoxime is altered in patients with impaired renal function; the half-life increases, while apparent plasma clearance and renal clearance decrease. The pharmacokinetics of cefpodoxime after oral administration of cefpodoxime proxetil are not affected by age.
Side effects of Cefpodoxime proxetil
Common side effects of Cefpodoxime proxetil include: headache, nausea, vomiting, diarrhoea, stomach pain, insomnia, vaginal swelling, redness, irritation, burning or itching. Severe may include: rash, itching, measles, difficulty breathing or swallowing, fever, sore throat, chills, or other signs of an infection coming back; swollen ankles or feet, dark urine, yellowing of the skin, easy bruising or bleeding, kidney problems. Seek medical attention if you have a serious adverse reaction
References
[1] BORIN M T. A review of the pharmacokinetics of cefpodoxime proxetil.[J]. Drugs, 1991. DOI:10.2165/00003495-199100423-00005.
You may like
See also
Lastest Price from Cefpodoxime proxetil manufacturers

US $10.00/ASSAYS2025-08-21
- CAS:
- 87239-81-4
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton

US $0.00/kg2025-04-22
- CAS:
- 87239-81-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg