Pharmacological research of AICAR
Introduction
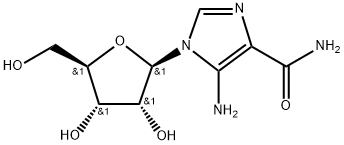
5-Aminoimidazole-4-carboxamide ribonucleoside (AICAR;Figure 1) has been one of the most commonly used pharmacological modulators of Activated adenosine monophosphate kinase (AMPK) activity.[1] AMPK is a heterotrimeric protein complex, with a catalytic α subunitand regulatory β and γ subunits. AMPK is an important regulator of cellular energy homeostasis as AMP allosterically activates the γ subunit. Further activation of AMPK occurs at phosphorylation sites on both the α and β subunits, through the action of AMPK kinases. AMPK phosphorylation activates peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1α) and PGC1β . Similarly, fatty acid uptake and metabolism is also upregulated both directly and indirectlyby AMPK. Therefore, AMPK may provide a mechanistic link between DOX-induced mitochondrial loss and dysfunction, leading to cardiotoxicity and we have previously proposed AMPK activation as a potential mechanism to prevent DOX-HF. AICAR is a prodrug, which is phosphorylated intracellularly to the active AMP analogue 5-aminoimidazole-4-carboxamide ribonucleotide (ZMP). As an AMPK activator, AICAR has been shown to increase mitochondrial biogenesis and fatty acid oxidation, though this has not previously been demonstrated in the context of DOX-HF.[2]
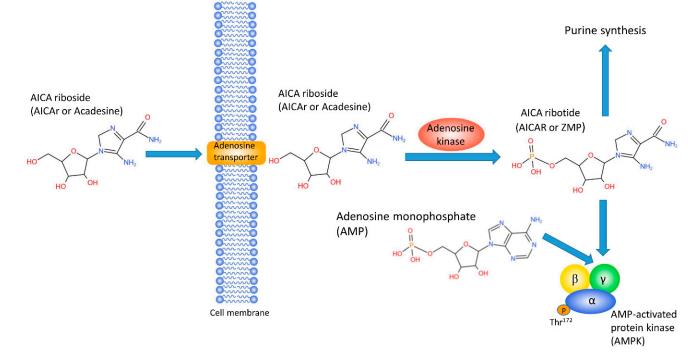
Metabolism
Since 1995, AICAR has been a valuable tool to identify metabolic pathways associated with AMPK activation in various tissues and cells in vivo and in vitro. In liver cells,AICAR was found to inhibit fatty acid and cholesterol synthesis, to increase fatty acid oxidation , and to inhibit gluconeogenesis by decreasing transcription of PEPCK. In skeletal muscle cells, AICAR was found to activate glycogen phosphorylase and increasegly cogenolysis , stimulate fatty acid oxidation and glucose uptake, and inhibit protein synthesis. All of these studies supported the role of AMPK as a central metabolic hub of the cell that is activated whenever the ratio of AMP/ATP is high and which acts to increase catabolic and inhibit anabolic processes.(Figure.1)[1]
Pharmacological research
1. AICAR confers prophylactic cardioprotection
Doxorubicin (DOX) is a widely used chemotherapeutic agent that can cause serious cardiotoxic side effects, leading to heart failure (HF). Impaired mitochondrial function is thought to be key factor driving progression into HF. Choksey et al. have previously shown in a rat model of DOX-HF that heart failure with reduced ejection fraction correlates with mitochondrial loss and dysfunction. Adenosine monophosphate-dependent kinase (AMPK) is a cellular energy sensor, regulating mitochondrial biogenesis and energy metabolism, including fatty acid oxidation. We hypothesised that AMPK activation could restore mitochondrial function and therefore be a novel cardioprotective strategy for the prevention of DOX-HF. Consequently, Choksey et al. set out to assess whether 5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside (AICAR), an activator of AMPK, could prevent cardiac functional decline in this chronic intravenous rat model of DOX-HF. In line with our hypothesis, AICAR improved cardiac systolic function. AICAR furthermore improved cardiac mitochondrial fatty acid oxidation, independent of mitochondrial number, and in the absence of observable AMPK-activation. In addition, Choksey et al. found that AICAR prevented loss of myocardial mass. RNAseq analysis showed that this may be driven by normalisation of pathways associated with ribosome function and protein synthesis, which are impaired in DOX-treated rat hearts. AICAR furthermore prevented dyslipidemia and excessive body-weight loss in DOX-treated rats, which may contribute to preservation of myocardial mass. Though it is unclear whether AICAR exerted its cardioprotective effect through cardiac or extra-cardiac AMPK-activation or via an AMPK-independent effect, these results show promise for the use of AICAR as a cardioprotective agent in DOX-HF to both preserve cardiac function and mass.[3]
2. AICAR stimulates mitochondrial biogenesis and BCAA catabolic enzyme expression in C2C12 myotubes
Type 2 diabetes is characterized by reduced insulin sensitivity, elevated blood metabolites, and reduced mitochondrial metabolism. Insulin resistant populations often exhibit reduced expression of genes governing mitochondrial metabolism such as peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α). Interestingly, PGC-1α regulates the expression of branched-chain amino acid (BCAA) metabolism, and thus, the consistently observed increased circulating levels of BCAA in diabetics may be partially explained by reduced PGC-1α expression. Conversely, PGC-1α upregulation appears to increase BCAA catabolism. PGC-1α activity is regulated by 5'-AMP-activated protein kinase (AMPK), however, only limited experimental data exists on the effect of AMPK activation in the regulation of BCAA catabolism. The present report examined the effects of the commonly used AMPK activator 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) on the metabolism and expression of several related targets (including BCAA catabolic enzymes) of cultured myotubes. C2C12 myotubes were treated with AICAR at 1 mM for up to 24 h. Mitochondrial and glycolytic metabolism were measured via oxygen consumption and extracellular acidification rate, respectively. Metabolic gene and protein expression were assessed via qRT-PCR and western blot, respectively. AICAR treatment significantly increased mitochondrial content and peak mitochondrial capacity. AICAR treatment also increased AMPK activation and mRNA expression of several regulators of mitochondrial biogenesis but reduced glycolytic metabolism and mRNA expression of several glycolytic enzymes. Interestingly, branched-chain alpha-keto acid dehydrogenase a (BCKDHa) protein was significantly increased following AICAR-treatment suggesting increased overall BCAA catabolic capacity in AICAR-treated cells. Together, these experiments demonstrate AICAR/AMPK activation can upregulate BCAA catabolic machinery in a model of skeletal muscle.[4]
3. AICAR Prevents and Reverses Diabetic Polyneuropathy
Diabetic peripheral neuropathy (DPN) is a common complication of diabetes in both Type 1 (T1D) and Type 2 (T2D). While there are no specific medications to prevent or treat DPN, certain strategies can help halt its progression. In T1D, maintaining tight glycemic control through insulin therapy can effectively prevent or delay the onset of DPN. However, in T2D, overall glucose control may only have a moderate impact on DPN, although exercise is clearly beneficial. Unfortunately, optimal exercise may not be feasible for many patients with DPN because of neuropathic foot pain and poor balance. Exercise has several favorable effects on health parameters, including body weight, glycemic control, lipid profile, and blood pressure. We investigated the impact of an exercise mimetic, 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), on DPN. AICAR treatment prevented or reversed experimental DPN in mouse models of both T2D and T1D. AICAR in high-fat diet (HFD-fed) mice increased the phosphorylation of AMPK in DRG neuronal extracts, and the ratio of phosphorylated AMPK to total AMPK increased by 3-fold (HFD vs. HFD+AICAR; p < 0.001). Phospho AMP increased the levels of dynamin-related protein 1 (DRP1, a mitochondrial fission marker), increased phosphorylated autophagy activating kinase 1 (ULK1) at Serine-555, and increased microtubule-associated protein light chain 3-II (LC3-II, a marker for autophagosome assembly) by 2-fold. Mitochondria isolated from DRG neurons of HFD-fed had a decrease in ADP-stimulated state 3 respiration (120 ± 20 nmol O2/min in HFD vs. 220 ± 20 nmol O2/min in control diet (CD); p < 0.001. Mitochondria isolated from HFD+AICAR-treated mice had increased state 3 respiration (240 ± 30 nmol O2/min in HFD+AICAR). However, AICAR's protection in DPN in T2D mice was also mediated by its effects on insulin sensitivity, glucose metabolism, and lipid metabolism. Drugs that enhance AMPK phosphorylation may be beneficial in the treatment of DPN.
Conclusions
Over the last 25 years, AICAR has been used in hundreds of studies as an activator of AMPK. The results of these initial studies pointed to the important roles of AMPK, and many of them have been later confirmed by studies in transgenic mice or by using models of cells with over expression or down-regulation of AMPK. However, AICAR accumulates in cells in millimolar concentrations and exerts many AMPK-independent or “off-target“effects so that allowances must be made for the possible use of AICAR. Although AICAR is no longer recommended as a specific AMPK agonist, mostly because there are many more specific activators of AMPK available nowadays, AICAR can be still useful in an initial screen to test for AMPK activation, especially when combined with other AMPK agonists and proper methods for AMPK downregulation. In addition, AICAR is still a highly promising pharmacological agent having many beneficial effects in metabolism,hypoxia, exercise, and cancer.[1]
References
1. Višnjić D, Lalić H, Dembitz V, Tomić B, Smoljo T. AICAr, a Widely Used AMPK Activator with Important AMPK-Independent Effects: A Systematic Review. Cells. 2021;10(5):1095. Published 2021 May 4. doi:10.3390/cells10051095
2. Chandrasekaran K, Choi J, Salimian M, Hedayat AF, Russell JW. Administration of AICAR, an AMPK Activator, Prevents and Reverses Diabetic Polyneuropathy (DPN) by Regulating Mitophagy. Int J Mol Sci. 2024;26(1):80. Published 2024 Dec 25. doi:10.3390/ijms26010080
3. Choksey A, Carter RD, Thackray BD, et al. AICAR confers prophylactic cardioprotection in doxorubicin-induced heart failure in rats. J Mol Cell Cardiol. 2024;191:12-22. doi:10.1016/j.yjmcc.2024.04.011
4.Hinkle JS, Rivera CN, Vaughan RA. AICAR stimulates mitochondrial biogenesis and BCAA catabolic enzyme expression in C2C12 myotubes. Biochimie. 2022;195:77-85. doi:10.1016/j.biochi.2021.11.004You may like
Related articles And Qustion
See also
Lastest Price from AICAR manufacturers

US $10.00-1.00/kg2025-10-31
- CAS:
- 2627-69-2
- Min. Order:
- 1kg
- Purity:
- 95%or98%
- Supply Ability:
- 300tons

US $0.00-0.00/box2025-10-17
- CAS:
- 2627-69-2
- Min. Order:
- 1box
- Purity:
- 99.99%
- Supply Ability:
- 10000