Mavacamten:Pharmacology,Mechanism and Adverse Effects
Introduction
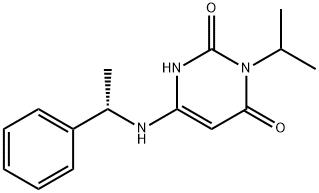
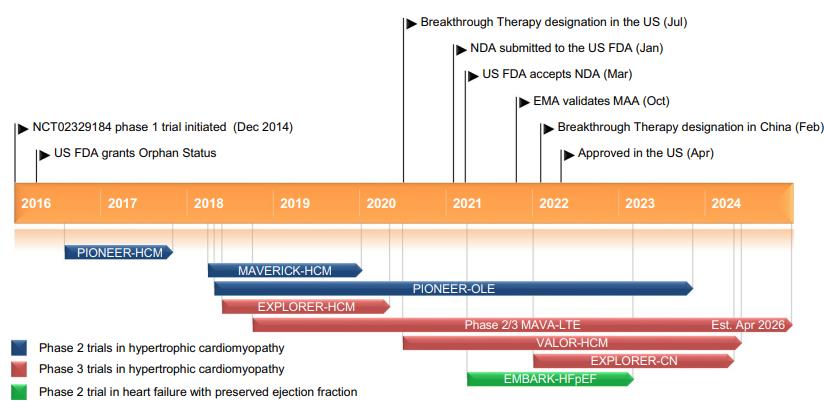
Mavacamten is a small-molecule allosteric and reversible inhibitor of cardiac myosin ATPase. It targets the sarcomere hypercontractility that is one of the characteristics of hypertrophic cardiomyopathy (HCM) and inhibits excessive myosin actin cross-bridge formation, shifting the overall myosin population towards an energy-sparing, recruitable, superrelaxed state. The chemical name of mavacamten is 3-(1-methylethyl)-6-[[(1S)-1-phenylethyl]amino]-2,4(1H,3H)-pyrimidinedione. The molecular formula is C15H19N3O2. The molecular weight is 273.33 g/mol. Mavacamten received its first approval on 28 April 2022 in the USA for the treatment of adults with symptomatic New York Heart Association (NYHA) class II-III obstructive HCM to improve functional capacity and symptoms and is under evaluation in the EU for the treatment of obstructive HCM.(FIgure 1) The recommended starting dose of mavacamten is 5 mg once daily without regard to food; allowable subsequent doses with titration are 2.5, 5, 10,or 15 mg once daily. Regular LVEF and Valsalva (provoked) LVOT gradient assessment is required for careful dose titration to achieve an appropriate target Valsalva LVOT gradient,while maintaining LVEF ≥ 50% and avoiding heart failure symptoms. Daily dosing takes weeks to reach steady-state drug levels and therapeutic effects, and genetic variation in metabolism and drug interactions can cause large differences in exposure. Algorithms for initiation and maintenance dosing, patient monitoring schedules, and guidance for treatment interruption or discontinuation are provided in the prescribing information and should be followed.[1]
Pharmacology of Mavacamten
Mavacamten is an allosteric and reversible inhibitor selective for cardiac myosin. It modulates the number of myosin heads that cross-bridge to actin during systole or diastole. Excess myosin–actin crossbridge formation and dysregulation of the super-relaxed state are mechanistic hallmarks of HCM. Mavacamten promotes an energy-sparing, recruitable, super-relaxed state. In patients with hypertrophic cardiomyopathy (HCM), this reduces dynamic LVOTO and improves cardiac filling pressures. Mavacamten is over 85% bioavailable following oral administration and reaches maximal concentration within 1 hour, which is increased to 4 hours when taken with a high-fat meal. It is extensively metabolized via cytochrome P450 (CYP) enzymes, primarily CYP2C19 (74%),CYP3A4 (18%), and CYP2C9 (8%). The mean half-life eliminations of mavacamten are 6 to 9 days in CYP2C19 normal metabolizers and 23days in CYP2C19 poor metabolizers. In patients with mild to moderate hepatic impairment (Child-Pugh class A or B), exposure is increased by up to 220% compared to that in patients with normal hepatic function;however, no dosage adjustment is necessary. The effect of severe hepatic impairment (Child-Pugh class C) is unknown, as it has not been studied. Mavacamten is excreted primarily in the urine (85%), with ≈3% beingunchanged drug.[2]
The AUC of mavacamten is increased up to 220% in patients with mild or moderate(Child-Pugh A and B) hepatic impairment. The effect of severe hepatic impairment (Child-Pugh C) is unknown. No clinically significant differences in pharmacokinetics were observed based on sex, race, ethnicity, age, or mild to moderate renal impairment (eGFR 30-89 mL/min/1.73m2). The effect of severe renal impairment (eGFR <30 mL/min/1.73 m2) or end stage renal disease requiring dialysis is unknown.[3]
Mechanism of Action of Mavacamten
To generate myocardial contraction, actin binding sites and the heads of myosin chains interact to allow for crossbridge formation and sarcomere shortening. Normally, approximately 40-50% of myosin heads are in an “off state” with very low energy utilization. However, in HCM only 15-20% of myosin heads are in the “off state” resulting in more ATP consumption,and more myosin heads are primed to interact with actin. Because more myosin heads are in the“on state,” more cross-bridge formations are present between myosin and actin throughout the entire cardiac cycle. This results in energetic inefficiencies, hyperdynamic contraction, and diastolic dysfunction. The ongoing hyperactivity within cardiac sarcomeres activates pathways that promote inflammation, hypertrophy, and fibrosis, which further lead to adverse cardiac remodeling.
Mavacamten is an allosteric, reversible actin-activated cardiac myosin ATPase inhibitor.Through this process, mavacamten reduces the number of myosin heads in the “on state”resulting in a reduced probability of myosin-actin cross-bridge formations throughout the cardiac cycle. By shifting cardiac myosin towards an energy-sparing, recruitable, super-relaxed state, this results in reduction in LVOTO and improves cardiac filling pressures.[3]
Adverse Effects and Precautions
Overall, studies to date have reported similar rates of adverse effects between mavacamten and placebo. The primary adverse effects to monitor for with mavacamten use are decreased LVEF and HF due to systolic dysfunction. These adverse effects are believed to be dose-related and have an intermediate onset, typically within 4 weeks of therapy initiation. Risk factors for mavacamten-associated HF include serious infection, uncontrolled tachyarrhythmia, concurrent use with disopyramide in combination with verapamil or diltiazem, concurrent use with certain moderate to strong CYP2C19 and/or CYP3A4 inhibitors, and discontinuation of certain moderate to strong CYP2C19 and/or CYP3A4 inducers. Additional adverse reactions include dizziness and syncope, which have been reported to occur at similar rates between the mavacamten and placebo groups in clinical trials. Mavacamten may also cause embryofetal toxicity and harm.[2]
References
[1].Keam SJ. Mavacamten: First Approval. Drugs. 2022;82(10):1127-1135. doi:10.1007/s40265-022-01739-7
[2].Chase Cole J, Benvie SF, DeLosSantos M. Mavacamten: A Novel Agent for Hypertrophic Cardiomyopathy. Clin Ther. 2024;46(4):368-373. doi:10.1016/j.clinthera.2024.02.007
[3].Schenk A, Fields N. Mavacamten-A Targeted Therapy for Hypertrophic Cardiomyopathy. J Cardiovasc Pharmacol. 2023;81(5):317-326. Published 2023 May 1. doi:10.1097/FJC.0000000000001416
You may like
See also

US $0.00-0.00/kg2025-04-21
- CAS:
- 1642288-47-8
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 500kg

US $1.00/g2025-04-21
- CAS:
- 1642288-47-8
- Min. Order:
- 1g
- Purity:
- 99.82%
- Supply Ability:
- 1000g