Polysaccharide
Polysaccharides are the sugars that can be hydrolyzed into many monosaccharide sugar molecules or its derivative, which are macromolecular compounds formed by the condensation and dehydration of 20 to tens of thousands of monosaccharide molecules. Some polysaccharides may be made by the condensation of one monosaccharide, such as starch, cellulose, which are called homopolysaccharide; Others may be formed by the condensation of different monosaccharides or derivatives thereof, such as hemicellulose, which are called hetero polysaccharides. The plants, animals and microorganisms in nature all contain polysaccharides. In addition to the free state, polysaccharide can also exist in the form combined with the protein. The common characters of plant polysaccharide include natural, health value and unique characteristics of the water-soluble colloids, which make the food have higher nutritional value, stable and palatable organizational structure, exquisite appearance, distinctive taste and extended shelf life. Natural plant polysaccharides are more secure, easily-absorbed, highly-utilized for human body. Water-soluble plant polysaccharides have excellent health value, such as konjac glucomannan that have the highest hydrated viscosity of soluble dietary fiber and have the healthy functions of full belly, reducing blood fat, lowering blood sugar and constipation. Plant polysaccharides do not provide calories, but have the balanced nutrition role for high-calorie, high-fat and high-protein diet. For hydrophilic plant polysaccharides, people used to call them the plant gum. Their hydrophilic colloid characteristics make the food production appear thickener, a gel (excipients), film formers, emulsifiers and other food stabilizers. The effective development and utilization of plant polysaccharides will help to improve the quality of human life and the level of human health, and enrich human food resources to create more economic value to the world food industry.
Starch is the polysaccharide stored in plants, and it is composed of two components, namely amylose and amylopectin. In the starch granules, amylose often only make up 20 to 28%, and the rest is amylopectin.250-300 D-glucose molecules link to a linear and then curl into a spiral via α-1,4-glycosidic bond to form the amylose, in which there is a non-reducing end and a reducing end. The molecular weight of amylose exist big difference, even from thousands to 150,000. Amylose can reacts with iodine to appear blue because the amylose is suspended in water, and its internal helical crimp of molecular is occupied by iodine (I2) .
Amylopectin structure is composed of 24-30 D-glucose molecules, which form short-chains viaα-1,4 glycosidic bond and then link short-chains into to bushes-like structure via α-1,6- glycosidic bond. The α-amylase in digestive tract of animals ,which present in saliva and pancreatic juice, can optionally be applied to work on α-1,4 glycosidic bond to hydrolyze linear amylose and form maltose and glucose. The β- amylase in plants can act on the non-reducing end of amylose to form maltose. Amylopectin can also be acted by α and β-amylase, but the α-1,4 glycosidic bond and α-1,6 glycosidic bond close to the branching point can’t be hydrolyzed by these enzymes. In the combined effect of amylase and α-1,6glucosidase, pullulan can be completely hydrolyzed to glucose and maltose.
Agarose is an important component of agar, which formed by the repeating condensation of D-galactose and 3,6 -anhydrogalactose. Agarose can dissolve in boiling water in large numbers, but when the temperature drop to below 40 ℃, agarose changes into insoluble gel. This agarose gel is commonly used as a support for the chromatography, but it is unstable in acid environment due to the cross-linked galactosidase is formed via hydrogen bonds but not the coordinate bonds.
- Structure:

- Chemical Name:Chitosan
- CAS:9012-76-4
- MF:C6H11NO4X2
- Structure:
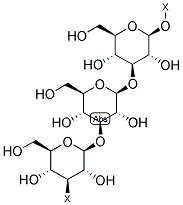
- Chemical Name:beta-(1,3)-D-Glucan
- CAS:9012-72-0
- MF:C18H30O14X2
- Chemical Name:Pectin
- CAS:9000-69-5
- MF:C6H12O6
- Structure:
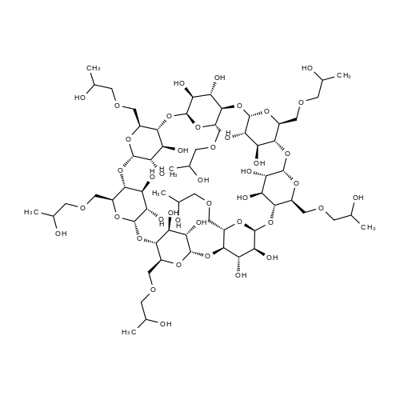
- Chemical Name:2-Hydroxypropyl-β-cyclodextrin
- CAS:128446-35-5
- MF:C63H112O42
- Structure:
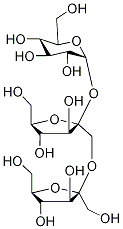
- Chemical Name:INULIN
- CAS:9005-80-5
- MF:C18H32O16
- Structure:
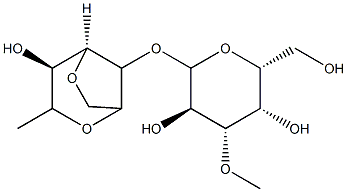
- Chemical Name:Agar
- CAS:9002-18-0
- MF:C14H24O9
- Structure:
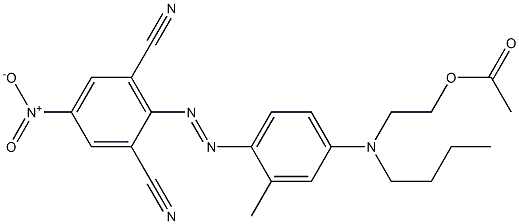
- Chemical Name:Ethyl cellulose
- CAS:9004-57-3
- MF:C23H24N6O4
- Structure:
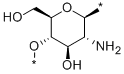
- Chemical Name:Chitin
- CAS:1398-61-4
- MF:C6H11NO4X2
- Structure:
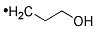
- Chemical Name:Hydroxypropyl cellulose
- CAS:9004-64-2
- MF:C3H7O*
- Structure:
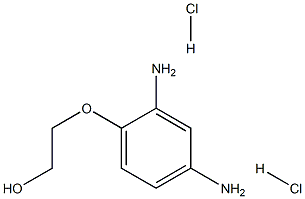
- Chemical Name:Xanthan gum
- CAS:11138-66-2
- MF:C8H14Cl2N2O2
- Structure:
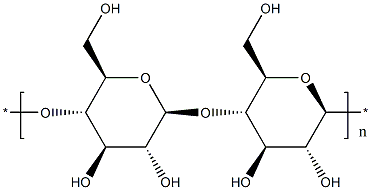
- Chemical Name:Microcrystalline cellulose
- CAS:9004-34-6
- MF:(C12H20O10)n
- Structure:
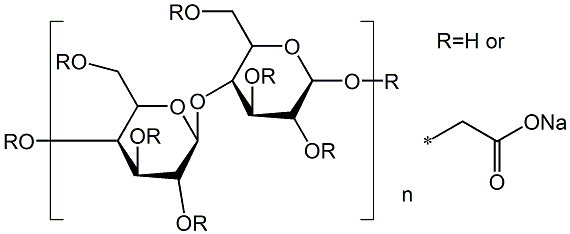
- Chemical Name:Sodium carboxymethyl cellulose
- CAS:9004-32-4
- MF:C6H7O2(OH)2CH2COONa
- Structure:
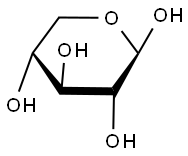
- Chemical Name:XYLAN
- CAS:9014-63-5
- MF:C5H10O5
- Chemical Name:CHITOSAN SALICYLATE
- CAS:84563-67-7
- MF:
- Chemical Name:CHITOSAN GLYCOLATE
- CAS:
- MF:
- Chemical Name:CARBOXYMETHYL BETA-CYCLODEXTRIN
- CAS:
- MF:
- Chemical Name:CROSCARMELLOSE SODIUM
- CAS:74811-65-7
- MF:Null
- Chemical Name:CHITOSAN SUCCINAMIDE
- CAS:
- MF:
- Chemical Name:Heparin lithium salt
- CAS:9045-22-1
- MF:n.a.
- Structure:
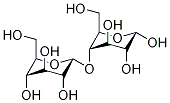
- Chemical Name:Maltodextrin
- CAS:9050-36-6
- MF:C12H22O11
- Chemical Name:Lipopolysaccharides
- CAS:
- MF:
- Structure:
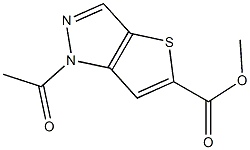
- Chemical Name:LIPOPHILIC SEPHADEX
- CAS:9041-37-6
- MF:C9H8N2O3S
- Chemical Name:SEPHADEX G-15
- CAS:11081-40-6
- MF:NULL
- Structure:
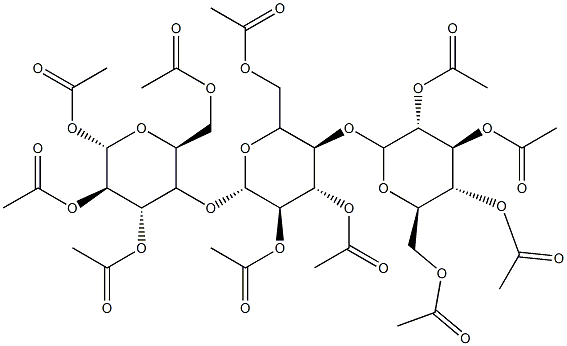
- Chemical Name:CELLULOSE TRIACETATE
- CAS:9012-09-3
- MF:C40H54O27
- Chemical Name:Agarose
- CAS:9012-36-6
- MF:C10H15N3O3
- Chemical Name:Starch soluble
- CAS:9005-84-9
- MF:(C6H10O5)n
- Chemical Name:Methyl 2-hydroxyethyl cellulose
- CAS:9032-42-2
- MF:C2H6O2·xCH4O·x
- Structure:
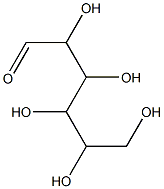
- Chemical Name:Carboxymethyl cellulose
- CAS:9000-11-7
- MF:C6H12O6
- Structure:
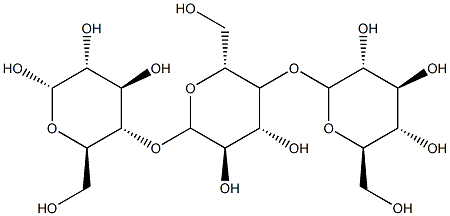
- Chemical Name:Dextrin
- CAS:9004-53-9
- MF:C18H32O16
- Chemical Name:GLYCOPROTEIN, ALPHA1-ACID HUMAN
- CAS:66455-27-4
- MF:NULL
- Structure:

- Chemical Name:CITRATE SYNTHASE
- CAS:9027-96-7
- MF:C6H5O7-3
- Structure:

- Chemical Name:GLYCOGEN
- CAS:9005-79-2
- MF:(C6H10O5)n
- Chemical Name:Cellulose Acetate Butyrate
- CAS:9004-36-8
- MF:N/A
- Chemical Name:CAROB GALACTOMANNAN
- CAS:11078-30-1
- MF:
- Structure:
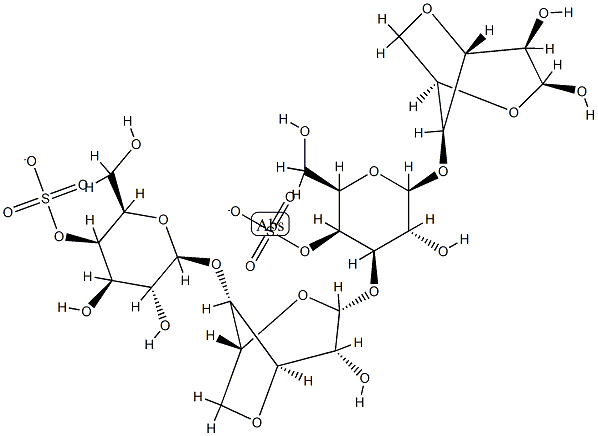
- Chemical Name:κ-Carrageenan
- CAS:11114-20-8
- MF:C24H36O25S2-2
- Chemical Name:Sulglycotide
- CAS:54182-59-1
- MF:
- Chemical Name:DEAE-cellulose
- CAS:9013-34-7
- MF:C20H23NO5
- Chemical Name:Cellulose acetate
- CAS:9004-35-7
- MF:[C6H7O2(OH)3-m(OOCCH3)m],m=0~3
- Chemical Name:AGAROSE
- CAS:39346-81-1
- MF:C24H38O19
- Chemical Name:SEPHADEX G-75
- CAS:37224-29-6
- MF:NULL
- Structure:
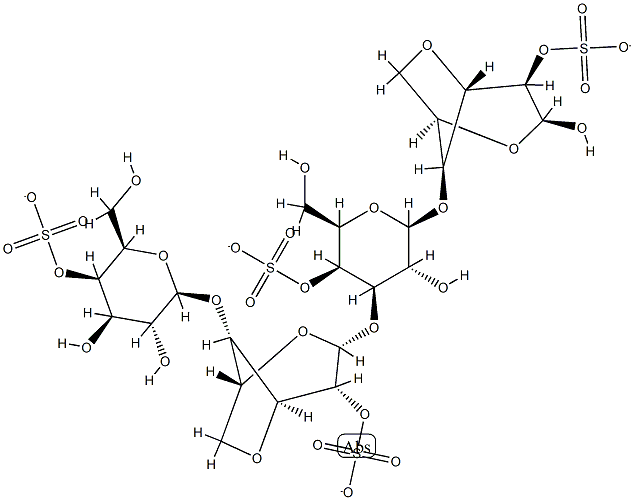
- Chemical Name:IRISH MOSS
- CAS:9062-07-1
- MF:C24H34O31S4-4
- Chemical Name:Pullulan
- CAS:
- MF:
- Structure:
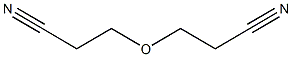
- Chemical Name:CYANOETHYL CELLULOSE
- CAS:9004-41-5
- MF:C6H8N2O
- Chemical Name:CARRAGEENAN
- CAS:9000-07-1
- MF:NULL
- Structure:
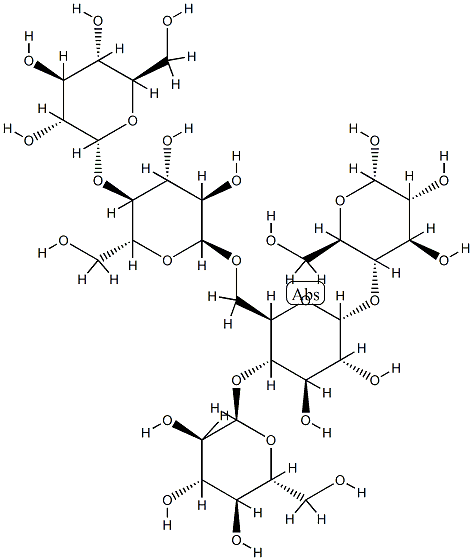
- Chemical Name:AMYLOPECTIN
- CAS:9037-22-3
- MF:C30H52O26
- Structure:
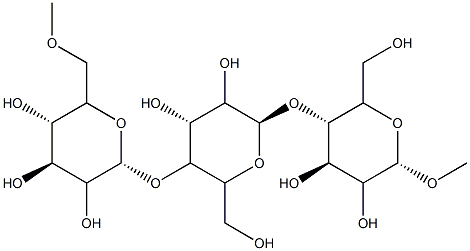
- Chemical Name:Pullulan
- CAS:9057-02-7
- MF:C20H36O16
- Structure:
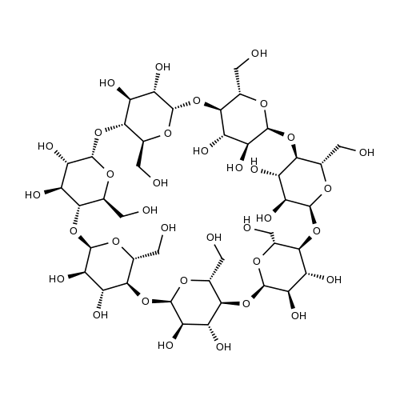
- Chemical Name:β-Cyclodextrin
- CAS:7585-39-9
- MF:C42H70O35
- Chemical Name:Starch
- CAS:9005-25-8
- MF:(C6H10O5)n
- Chemical Name:SP SEPHADEX
- CAS:61840-62-8
- MF:N/A
- Structure:
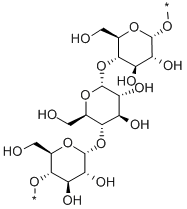
- Chemical Name:AMYLOSE
- CAS:9005-82-7
- MF:C18H30O16X2
- Chemical Name:CELLULOSE ACETATE PROPIONATE
- CAS:9004-39-1
- MF:C76H114O49
- Structure:
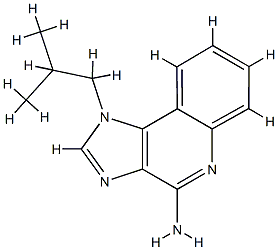
- Chemical Name:Hydroxypropyl methylcellulose phthalate
- CAS:9050-31-1
- MF:C14H16N4
- Chemical Name:Dextran Sulfate Sodium Salt
- CAS:9011-18-1
- MF:(C6H7Na3O14S3)n
- Structure:
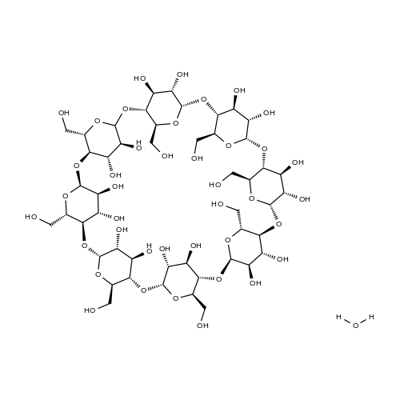
- Chemical Name:GAMMA-CYCLODEXTRIN HYDRATE
- CAS:91464-90-3
- MF:C48H82O41
- Chemical Name:SEPHADEX(R) G-200 SUPERFINE, FOR CHROMAT OGRAPHY
- CAS:
- MF:
- Chemical Name:CARBOXYMETHYL SEPHADEX
- CAS:62886-59-3
- MF:
- Chemical Name:SEPHADEX G-100
- CAS:9050-94-6
- MF:NULL
- Structure:
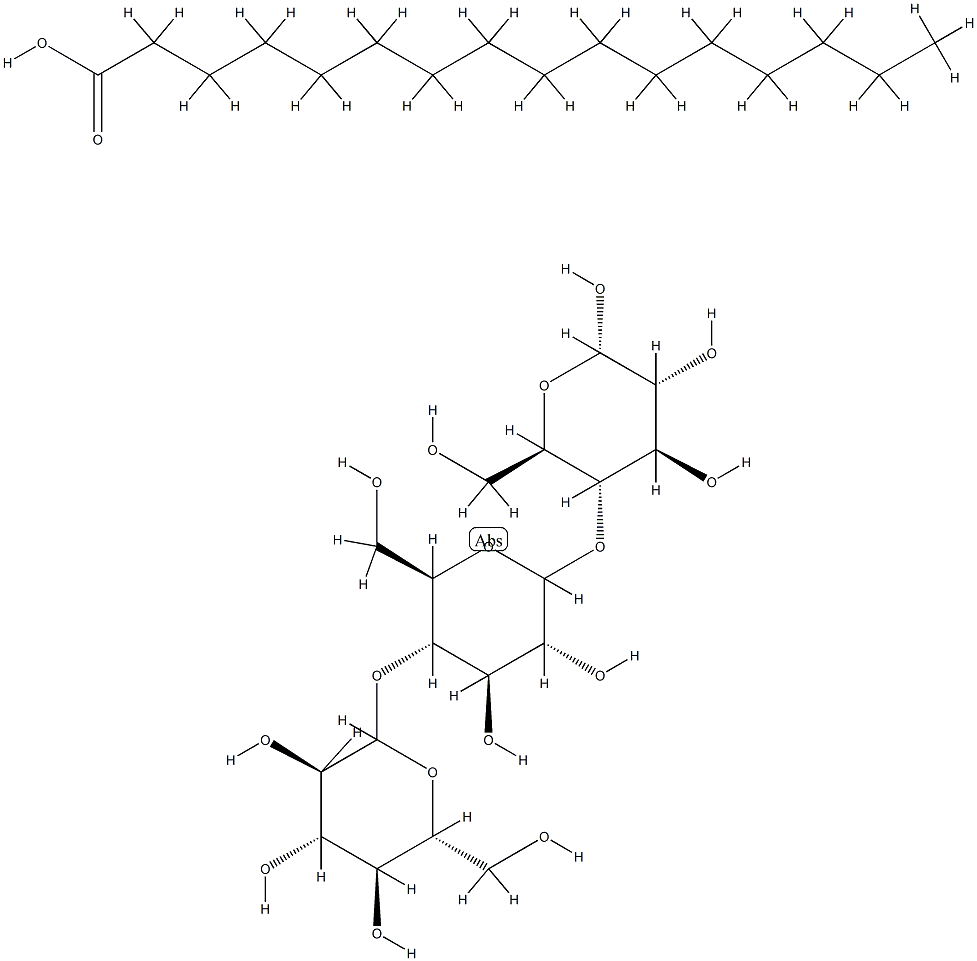
- Chemical Name:DEXTRIN PALMITATE
- CAS:83271-10-7
- MF:C34H64O18
- Chemical Name:CARBOXYMETHYL SEPHADEX
- CAS:9047-08-9
- MF:
- Structure:
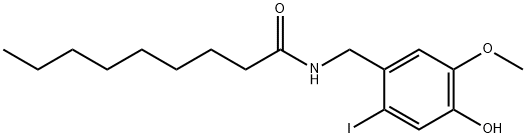
- Chemical Name:6-IODONORDIHYDROCAPSAICIN
- CAS:859171-97-4
- MF:C17H26INO3