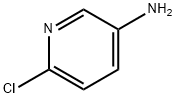
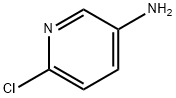
Uses
5-Amino-2-chloropyridine was used in the synthesis of [
2H
5]2-amino-l-methyl-6-phenylimidazo[4,5-
b]pyridine. It was used in identification and evaluation of molecularly imprinted polymers for the selective removal of potentially genotoxic aminopyridine impurities from pharmaceuticals.
General Description
5-Amino-2-chloropyridine undergoes Suzuki-Miyaura coupling with sterically hindered 2,6-dimethylphenylboronic acid. It undergoes facile temperature dependent displacement of chloride by bromide via Sandmeyer reaction to yield 2,5-dibromopyridine.
Synthesis
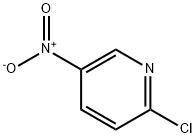
General method: 2-chloro-5-nitropyridine (1 eq.), hexafluoroisopropanol (HFIP, 10 eq.) and iron powder (5 eq.) were added to the reaction tube and mixed. Subsequently, 2N aqueous hydrochloric acid solution was slowly added to the reaction mixture. The reaction mixture was stirred at room temperature for 30 minutes and then neutralized with saturated aqueous sodium bicarbonate (NaHCO3). The organic layer was extracted three times with saturated aqueous sodium bicarbonate solution and combined. The organic layers were subsequently washed with brine, dried over anhydrous sodium sulfate (Na2SO4), filtered, and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography to afford the target product 2-chloro-5-aminopyridine.
References
[1] Bulletin des Societes Chimiques Belges, 1988, vol. 97, # 1, p. 51 - 54
[2] Organic Letters, 2009, vol. 11, # 6, p. 1345 - 1348
[3] ACS Catalysis, 2014, vol. 4, # 6, p. 1777 - 1782
[4] New Journal of Chemistry, 2015, vol. 39, # 11, p. 8498 - 8504
[5] Tetrahedron Letters, 2017, vol. 58, # 37, p. 3646 - 3649