Hydrochloric acid
- Product NameHydrochloric acid
- CAS7647-01-0
- CBNumberCB7421538
-
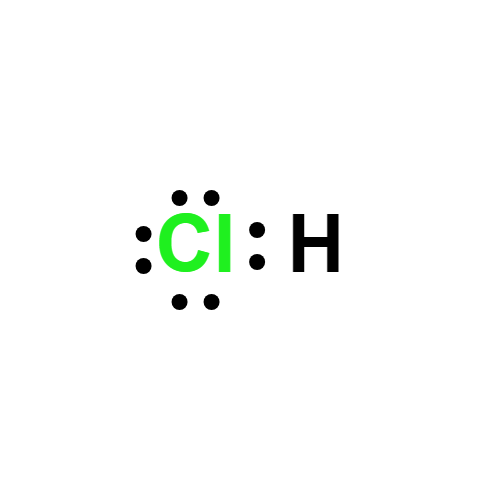
MFClH
Lewis structure
- MW36.46
- EINECS231-595-7
- MDL NumberMFCD00011324
- MOL File7647-01-0.mol
Chemical Properties
| Melting point | -35 °C |
| Boiling point | >100 °C (lit.) |
| Density | 1.2 g/mL at 25 °C (lit.) |
| vapor density | 1.3 (vs air) |
| vapor pressure | 613 psi ( 21.1 °C) |
| Flash point | 10℃ (tag closed test) |
| refractive index | 1.3535 |
| storage temp. | Store at +2°C to +25°C. |
| solubility | H2O: soluble |
| form | liquid |
| pka | -7(at 25℃) |
| color | Light Yellow |
| Specific Gravity | 1.19 |
| PH | 3.01(1 mM solution);2.04(10 mM solution);1.08(100 mM solution); |
| Odor | Sharp, irritating odor detectable at 0.25 to 10 ppm |
| explosive limit | 2-13.4% (v/v) 2-Propanol) |
| biological source | synthetic |
| Viscosity | 1.7mm2/s |
| Water Solubility | miscible |
| Sensitive | Air & Light Sensitive |
| Merck | 14,4780 |
| Exposure limits | Ceiling limit 5 ppm (~ 7 mg/m3). |
| Dielectric constant | 4.6(20℃) |
| Stability | Stable. Incompatible with alkalies, most metals. Avoid contact with water. |
| CAS DataBase Reference | 7647-01-0(CAS DataBase Reference) |
| FDA 21 CFR | 182.1057; 582.1057; 310.545 |
| Substances Added to Food (formerly EAFUS) | HYDROCHLORIC ACID |
| SCOGS (Select Committee on GRAS Substances) | Hydrochloric acid |
| EWG's Food Scores | 2-3 |
| FDA UNII | QTT17582CB |
| ATC code | A09AB03,B05XA13 |
| IARC | 3 (Vol. 54) 1992 |
| NIST Chemistry Reference | Hydrogen chloride(7647-01-0) |
| Pesticides Freedom of Information Act (FOIA) | Hydrogen chloride |
| EPA Substance Registry System | Hydrochloric acid (7647-01-0) |
| UNSPSC Code | 47131831 |
| NACRES | NB.21 |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H290 | |||||||||
| Precautionary statements | P234-P390 | |||||||||
| Hazard Codes | T,C,F,Xi,F+,Xn | |||||||||
| Risk Statements | 36/37/38-37-34-35-23-20-11-67-66-22-19-12-10-40-20/22-39/23/24/25-23/24/25-41-37/38 | |||||||||
| Safety Statements | 26-45-36/37/39-9-33-29-16-46-36/37-39 | |||||||||
| RIDADR | UN 2924 3/PG 2 | |||||||||
| OEL | Ceiling: 5 ppm (7 mg/m3) | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | MW4025000 | |||||||||
| F | 3 | |||||||||
| Autoignition Temperature | 425°C (2-Propanol) | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2806 10 00 | |||||||||
| DOT Classification | 2.3, Hazard Zone C (Gas poisonous by inhalation) | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | I | |||||||||
| Hazardous Substances Data | 7647-01-0(Hazardous Substances Data) | |||||||||
| Toxicity | LC50 (30 min) in mice, rats: 2142, 5666 ppm (Darmer) | |||||||||
| IDLA | 50 ppm | |||||||||
| NFPA 704: |
|
Hydrochloric acid Chemical Properties,Usage,Production
History
Basilus Valentinus of Italy was first to isolate the acid and reported it under the name spiritus salis in the fifteenth century. Glauber prepared this acid by the reaction of sulfuric acid with common salt in 1648. Lavoisier proposed the name muriatic acid in 1789 after muriate, the term referring to a chlorine-containing inorganic substance. Sir Humphrey Davy proved the gas was composed of only hydrogen and chlorine in 1810. Subsequently, the gas was named hydrogen chloride.Dilute hydrochloric acid occurs in the stomachs of mammals. Gaseous hydrogen chloride occurs in trace concentrations in the atmosphere.
Uses
Hydrochloric acid is one of the most important industrial chemicals and has numerous applications. Both anhydrous hydrogen chloride and aqueous acid are used to produce a large number of chloride salts. The acid also is a common laboratory reagent. Some major applications of hydrochloric acid include processing of ores and extraction of metals from their minerals; in metal cleaning, particularly in steel pickling to dissolve oxide impurities; production of alumina, titanium dioxide, and other metal oxides by various hydrometallurgical processes; production of hydrogen; synthesis of chlorine dioxide; removal of heavy metal impurities from carbon black; activation of bentonite clays; etching of concrete surfaces for finishing operations; and as a catalyst in several organic reactions such as inversion of sugar, hydrolysis of starch to obtain sugar syrup, and esterification of aromatic acids.Anhydrous hydrogen chloride gas is used to produce phosphonium chloride, PH4Cl, which is a flame retardant for cotton textiles. Other major applications include manufacture of a number of high purity metal chlorides, ammonium chloride, chlorosulfuric acid; recovery of waste metals; preparation of alkyl chlorides and chloroacetic acids; and as a chlorinating agent in organic syntheses.
Rubber hydrochloride, which results from the treatment of natural rubber with hydrogen chloride, can be cast in film from solutions. Such rubber hydrochloride films provide a strong, water resistant packaging material for meats and other foods, paper products, and textiles.
Production
Hydrochloric acid can be produced by several methods. It is obtained from the reaction of sodium chloride and sulfuric acid in a cast iron retort at elevated temperature. Although reaction starts at 150°C, the complete reaction occurs at about 600°C:2NaCl + H2SO4→ Na2SO4 + 2HCl
Hydrochloric acid also is made by the Hargreaves process in which a mixture of salt, sulfur dioxide, oxygen, and water are heated at elevated temperatures, between 430 to 540°C. The reaction is exothermic and becomes selfsustaining:
4NaCl + SO2 + O2 + 2H2O→ 2Na2SO4 + 4HCl
Hydrochloric acid may be produced by hydrolysis of metal chlorides such as titanium(IV) chloride:
TiCl4 + 2H2O →TiO2 + 4HCl
High purity HCl for commerce is made directly from hydrogen and chlorine:
H2 + Cl2→ 2HCl
The above reaction is highly exothermic. The stoichiometric proportion of gaseous mixture at equilibrium flame temperature is cooled to 200°C, whereupon the elements combine rapidly to form HCl with over 99% yield.
HCl also may be prepared by several other methods including thermal dissociation of aluminum chloride hexahydrate, AlCl3•6H2O, and as a by-product of manufacturing many organic compounds.
Crude HCl gas mixture may be purified by cooling and drying over concentrated sulfuric acid, which also removes organic unsaturated contaminants.
Organic contaminants may be removed further by adsorption over molecular sieves, polystyrene foam, active carbon, or scrubbing with a high-boiling point organic liquid.
Commercial grade, concentrated hydrochloric acid is about 37.5% HCl by weight and has a normality of 12 and specific gravity 1.19.
Hydrogen chloride gas may be stored in steel cylinders free of contaminants. Monel, pure nickel, or its alloy, inconel, may also be used for storage and transportation up to 500°C. Hydrochloric acid may be stored in glass bottles or in containers made up of tantalum or tantalum-molybdenum alloys, or other alloys of zirconium, molybdenum, and tungsten.
Reference
Handbook of Inorganic ChemicalsDescription
A water solution of hydrogen chloride of varied concentrations. It is a clear, colorless or slightly yellowish, corrosive liquid having a pungent odor. It is miscible with water and with alcohol. Concentrations of hydrochloric acid are expressed in percent by weight, or may be expressed in Baume degrees (Be0) from which percentages of hydrochloric acid and specific gravities may readily be derived. The usually available concentrations are 18°, 20°, 22°, and 23° Be. Concentrations above 13° Be (19.6%) fume in moist air, lose hydrogen chloride, and create a corrosive atmosphere. Because of these characteristics, suitable precautions must be observed during sampling and analysis to prevent losses. Note: Hydrochloric acid is produced by various methods that might impart trace amounts of organic compounds as impurities. The manufacturer, vendor, or user is responsible for identifying the specific organic compounds that are present and for meeting the requirements for organic compounds. Methods are provided for their determination. In applying the procedures any necessary standards should be used to quantitate the organic compounds present in each specific product.Chemical Properties
Hydrochloric acid occurs as a clear, colorless, fuming aqueous solution of hydrogen chloride, with a pungent odor.The JP XV specifies that hydrochloric acid contains 35.0–38.0% w/w of HCl; the PhEur 6.0 specifies that hydrochloric acid contains 35.0–39.0% w/w of HCl; and the USP32–NF27 specifies that hydrochloric acid contains 36.5–38.0% w/w of HCl.
Chemical Properties
Hydrochloric acid, or hydrogen chloride, is either a colorless liquid with a pungent odor, or a colorless to slightly yellow gas that can be shipped as a liquefi ed compressed gas. The acid is used in the production of fertilizers, dyes, dyestuffs, artifi cial silk, and paint pig- ments, and in refi ning edible oils and fats. Hydrochloric acid is also used in electroplating, leather tanning, ore refi ning, soap refi ning, petroleum extraction, and pickling of metals, and is used in the photographic, textile, and rubber industries. In addition, hydrochloric acid is used as an antiseptic in toilet bowls against animal pathogenic bacteria, and in food processing as a starch modifi er.Chemical Properties
Hydrogen chloride, HCl, is a colorless, fuming, highly toxic gas that is soluble in water, alcohol, and ether. It is used in polymerization, isomerization, and the synthesis of vinyl chloride and alkyl chloride.Physical properties
Colorless gas; sharp pungent odor; fumes in air; nonflammable; refractiveindex of gas at 0°C 1.000446; density of the gas 1.639 g/L (1.268 times heav-ier than air); density of liquid at -155°C 1.045 g/cm3; density of solid at-192°C 1.507 g/cm3; liquefies at -85.05°C to a colorless liquid; freezes to awhite crystalline solid at -114.22°C; critical temperature 51.55°C; criticalpressure 82.01 atm; critical volume 81 cm3/mol; triple point -114.25°C; dielec-tric constant at 25°C 1.0046; electrical conductivity 35.0 micromho/cm at-87.6°C; highly soluble in water 42.02 g/100 g solution (or 72.47 g/100 g water)at 20°C and 1 atm; soluble in alcohols and ethers (47.0 g and 24.9 g/100 g solu-tion at 20°C in methanol and ether, respectively.)Hydrochloric acid is a colorless to yellowish liquid (the yellow colorationmay be due to traces of iron, chlorine or organics contaminants); fumes in air;refractive index of 1.0 N solution 1.3417; density of commercial concentratedacid (37.8 g/100g solution) 1.19 g/mL, and constant boiling solution (20.22g/100g solution) 1.096 g/mL at 25°C; forms a constant boiling azeotrope withwater at HCl concentration 20.22%; the azeotrope boils at 108.6°C; severalmetal chlorides can be salted out of their aqueous solutions by addition ofHCl; the addition of CaCl2can break the azeotrope; the pH of the acid at 1.0,0.1 and 0.01 N concentrations are 0.10, 1.1, and 2.02, respectively; a 10.0 Msolution ionizes to 92.6% at 18°C.
History
Hydrochloric acid is a strong, corrosive acid that results when the gas hydrogen chloride dissolves in water.Ancient alchemists prepared hydrochloric acid and Jabbar ibn Hayyan, known in Latin as Geber (721–815), is credited with its discovery around the year 800. The original method of preparation involved reacting salt with sulfuric acid, producing sodium hydrogen sulfate and hydrogen chloride gas. The hydrogen chloride gas is captured and dissolved in water to produce hydrochloric acid. Hydrochloric acid was formerly called muriatic acid. Terms such as muriatic and muriate were used in association with chloride substances before the discovery and nature of chlorine were fully understood. The Latin term muriaticus means pickled from muri, which is the Latin term for brine. Chlorides were naturally associated with seawater salt solutions, as chloride is the principal ion in seawater.Uses
Hydrochloric acid is one of the most widely used acids and a common laboratory reagent. It is used in the manufacture of chlorides, in the pickling and cleaning of metal products, as a processing agent for manufacturing various food products, as a cleaning agent, in organic synthesis, and for neutralizing alkalies.Hydrogen chloride is a fire-effluent gas.Firefighters are frequently exposed to significant concentrations of HCl (Brandt-Raufet al. 1988). Large amounts of HCl arereleased from the oxidative thermal degradation of polyvinyl chloride (PVC)-derivedfiberglass, cotton, and jute brattices in mines.At 250°C (482°F) its concentration is foundto be >5 ppm (De Rosa and Litton 1986).The gas is absorbed by water droplets,entrapped in soot particles, causing risk ofexposure of the acid to the eyes, throat,and lungs of mine workers. Stack emissionsof HCl can result from burning plastic-richwastes (e.g., hospital wastes) (Powell 1987).Emissions of 1.0–1.6 g HCl/kg waste havebeen reported (Allen et al. 1986)..
Uses
Pickling is a metal treatment process used to prepare metal surfaces for subsequent processing such as galvanizing or extrusion. In the iron industry, pickling involves immersing iron and steel products in vats of diluted hydrochloric acid. This removes oxides, dirt, and grease. Oil well acidizing involves injecting hydrochloric acid down well holes to dissolve limestone and carbonate formations. This expands existing fissures and creates new fissures to open channels for oil extraction.Hydrochloric acid is also used extensively in pharmaceuticals and the food industry. When it is listed after a drug name, the drug was produced by combining a free base and hydrochloric acid to produce a hydrochloride salt. Drugs delivered as hydrochloride salts rather than free bases are more soluble in water than free forms of the drugs, tend to be more stable, are solids, and are often more compatible with the chemistry of the digestive system. In the food industry it is used in the production of gelatin and sodium glutamate, to convert cornstarch to syrup, to refine sugar, and as an acidulant.
Uses
In the production of chlorides; refining ore in the production of tin and tantalum; for the neutralization of basic systems; as laboratory reagent; hydrolyzing of starch and proteins in the preparation of various food products; pickling and cleaning of metal products; as catalyst and solvent in organic syntheses. Also used for oil- and gas-well treament and in removing scale from boilers and heat-exchange equipment. Pharmaceutic aid (acidifier).Uses
Hydrochloric Acid is an acid that is the aqueous solution of hydro- gen chloride of varying concentrations. it is miscible with water and with alcohol. it is used as an acidulant and neutralizing agent.Definition
A colorless fuming liquid made by adding hydrogen chloride to water:HCl(g) + H2O1. → H3O+(aq) + Cl-(aq)
Dissociation into ions is extensive and hydrochloric acid shows the typical properties of a strong acid. It reacts with carbonates to give carbon dioxide and yields hydrogen when reacted with all but the most unreactive metals. Hydrochloric acid is used in the manufacture of dyes, drugs, and photographic materials. It is also used to pickle metals, i.e. clean the surface prior to electroplating. Hydrochloric acid donates protons with ease and is the strongest of the hydrohalic acids. The concentrated acid is oxidized to chlorine by such agents as potassium manganate(VII) and manganese( IV) oxide.
Production Methods
Hydrochloric acid is an aqueous solution of hydrogen chloride gas produced by a number of methods including: the reaction of sodium chloride and sulfuric acid; the constituent elements; as a by-product from the electrolysis of sodium hydroxide; and as a by-product during the chlorination of hydrocarbons.Definition
hydrogen chloride: A colourlessfuming gas, HCl; m.p. –114.8°C; b.p.–85°C. It can be prepared in the laboratoryby heating sodium chloridewith concentrated sulphuric acid(hence the former name spirits ofsalt). Industrially it is made directlyfrom the elements at high temperatureand used in the manufacture ofPVC and other chloro compounds. Itis a strong acid and dissociates fullyin solution (hydrochloric acid).Definition
ChEBI: A mononuclear parent hydride consisting of covalently bonded hydrogen and chlorine atoms.Production Methods
The traditional method of preparation of hydrochloric acid is the reaction of metal chlorides, especially sodium chloride with sulfuric acid (see the first reaction described). Hydrochloric acid is also produced by direct synthesis from its elements. In the chlorine-alkali industry, electrochemical reactions produce elemental chlorine and hydrogen, which can then be combined to give hydrogen chloride: Cl2(g) + H2(g) 2HCl(g). Hydrogen chloride is then dissolved in water to produce hydrochloric acid. By far, the most common method of producing hydrochloric acid involves its production as a by-product in chlorination reactions. This has curtailed this source of hydrochloric acid. The production of other common industrial organic chemicals such as Teflon, perchloroethylene, and polyvinyl chloride result in the production of hydrogen chloride. The production of hydrochloric acid in polyvinyl chloride production takes place when ethylene is chlorinated: C2H4(g) + Cl2(g) C2H4Cl2(g) C2H4Cl2(g)(g) C2H3Cl(g) + HCl(g).Air & Water Reactions
Fumes strongly in moist air. Soluble in water with evolution of heat.Reactivity Profile
ANHYDROUS HYDROGEN CHLORIDE is an anhydrous (no water) strong acid. Reacts rapidly and exothermically with bases of all kinds (including amines and amides). Reacts exothermically with carbonates (including limestone and building materials containing limestone) and hydrogen carbonates to generate carbon dioxide. Reacts with sulfides, carbides, borides, and phosphides to generate toxic or flammable gases. Reacts with many metals (including aluminum, zinc, calcium, magnesium, iron, tin and all of the alkali metals) to generate flammable hydrogen gas. Reacts violently with acetic anhydride, 2-aminoethanol, ammonium hydroxide, calcium phosphide, chlorosulfonic acid, 1,1-difluoroethylene, ethylenediamine, ethyleneimine, oleum, perchloric acid, b-propiolactone, propylene oxide, silver perchlorate/carbon tetrachloride mixture, sodium hydroxide, uranium(IV) phosphide, vinyl acetate, calcium carbide, rubidium carbide, cesium acetylide, rubidium acetylide, magnesium boride, mercury(II) sulfate [Lewis]. Undergoes a very energetic reaction with calcium phosphide [Mellor 8:841(1946-1947)]. Corrosive to metals and tissues and irritating to the eyes and respiratory system. Corrodes galvanized or copper-alloy metals (brass, bronze); fittings of stainless steel or mild or cast steel must therefore be used. Reacts with calcium carbide with incandescence [Mellor 5:862(1946-1947]. Absorption on mercuric sulfate becomes violent at 125°C. [Mellor 2, Supp. 1:462(1956)].Hazard
Toxic by inhalation, strong irritant to eyes and skin. Questionable carcinogen.Health Hazard
Gas concentrations of 50 to 100 ppm are tolerable for 1 hour. Concentrations of 1,000 to 2,000 ppm are dangerous, even for brief exposures. More severe exposures will result in serious respiratory distress and prolonged exposures will result in death. Mists of hydrochloric acid are considered less harmful than anhydrous hydrochloric acid, because droplets have no dehydrating action. Individuals with respiratory problems and digestive diseases may be adversely affected by low level exposures to the gas or mist.Health Hazard
Concentrated hydrochloric acid is a corrosivesubstance that can cause severe burns.Spilling into the eyes can damage vision.Ingestion can produce corrosion of themouth, gastrointestinal tract, and stomach,and diarrhea.Hydrogen chloride is a toxic gas with acharacteristic pungent odor. Inhalation cancause coughing, choking, and irritation ofthe mucous membranes. Exposure to concentrations at >5 ppm in air can be irritating and disagreeable to humans (Patty 1963;ACGIH 1986). A short exposure to 50 ppmmay cause irritation of the throat. Workersexposed to hydrochloric acid were found tosuffer from gastritis and chronic bronchitis(Fairhall 1957).
Rats exposed continuously to a hydrogen chloride atmosphere died after physicalincapacitation (Crane et al. 1985). Hartzelland coworkers (1987) have studied thetoxicological effects of smoke containinghydrogen chloride in fire gases. The lethality of PVC smoke was high but not entirelydue to the hydrogen chloride produced. Postexposure death in rats was observed afterpulmonary irritation caused by high concentration of HCl. Lethality in the presenceof carbon monoxide may be additive. Inanother paper, Hartzell and associates (1988)reported that guinea pigs were three timesas sensitive as rats to HCl exposure. HClproduced bronchoconstriction in animals andshowed additive toxicity with CO at relatively high concentrations of the latter.
Health Hazard
Hydrochloric acid and hydrogen chloride gas are highly corrosive substances that may cause severe burns upon contact with any body tissue. The aqueous acid and gas are strong eye irritants and lacrimators. Contact of conc hydrochloric acid or concentrated HCl vapor with the eyes may cause severe injury, resulting in permanent impairment of vision and possible blindness, and skin contact results in severe burns. Ingestion can cause severe burns of the mouth, throat, and gastrointestinal system and can be fatal. Inhalation of hydrogen chloride gas can cause severe irritation and injury to the upper respiratory tract and lungs, and exposure to high concentrations may cause death. HCl gas is regarded as having adequate warning propertiesHydrogen chloride has not been found to be carcinogenic or to show reproductive or developmental toxicity in humans
Health Hazard
Exposures to hydrochloric acid cause severe health effects and corrosive reactions. Concentrated hydrochloric acid (fuming hydrochloric acid) forms acidic mists. Both the mist and the solution have a corrosive effect on human tissue, with the potential to damage the respiratory organs, eyes, skin, and intestines. Inhalation of vapors can cause coughing, choking, infl ammation of the nose, throat, and upper respiratory tract, and in severe cases, pulmonary edema, circulatory failure, and death. Accidental ingestion and/or swallow- ing of hydrochloric acid at workplaces causes immediate pain and burns of the mouth, throat, esophagus, and gastrointestinal tract. It also causes nausea, vomiting, and diar- rhea, and in severe cases, death. Any kind of contact of the skin surfaces to hydrochloric acid causes redness, pain, and severe skin burns. Concentrated solutions of hydrochloric acid cause deep ulcers and discolor the skin. Vapors of hydrochloric acid cause irritat- ing effects to the eyes and eye damage, leading to severe burns and permanent eye dam- age. Long-term exposures to concentrated vapors of hydrochloric acid cause erosion of the teeth. Occupational workers and persons with pre-existing skin disorders or eye disease are more susceptible to the effects of hydrochloric acid.Fire Hazard
Noncombustible, but contact with metals may produce highly flammable hydrogen gas.Fire Hazard
Fire may produce irritating or poisonous gases. Containers may explode in heat of fire. At high temperatures, Hydrochloric acid decomposes into hydrogen and chlorine. The following materials should be avoided: Mercuric sulfate -- violent reaction with gaseous hydrochloric acid at 250F. Sodium -- reacts vigorously with gaseous hydrochloric acid. Acetic anhydride, 2-aminoethanol, ammonium hydroxide, chlorosulfonic acid, ethylene diamine, ethyleneimine, oleum, propiolactone, sodium hydroxide, sulfuric acid, and vinyl acetate -- increase in temperature and pressure when mixed with hydrochloric acid. Calcium phosphide -- energetic reaction with hydrochloric acid. Silver perchlorate and carbon tetrachloride -- when mixed in combination with hydrochloric acid forms a compound that detonates at 105F. Formaldehyde -- when mixed with hydrochloric acid forms a human carcinogen. Material reacts violently with bases and is corrosive with the generation of heat. Reacts with base metals, forming combustible gas (hydrogen). Reacts violently with strong oxidants forming toxic gas (chlorine). Avoid heat; at high temperatures Hydrochloric acid will decompose into hydrogen and chlorine.Flammability and Explosibility
Noncombustible, but contact with metals may produce highly flammable hydrogen gas.reaction suitability
reagent type: derivatization reagentreaction type: Alkylations
Pharmaceutical Applications
Hydrochloric acid is widely used as an acidifying agent, in a variety of pharmaceutical and food preparations. It may also be used to prepare dilute hydrochloric acid, which in addition to its use as an excipient has some therapeutic use, intravenously in the management of metabolic alkalosis, and orally for the treatment of achlorhydria.Industrial uses
Hydrochloric acid (HCl) is soluble in water andis a strong mineral acid made by the action ofsulfuric acid on common salt, or as a byproductof the chlorination of hydrocarbons such asbenzene.HCl is used to some extent in pickling of metal prior to porcelain enameling.Industrial uses
Hydrochloric acid (HCl) is a highly corrosive liquid, emitting a pungent odor and fumes in moist air. Concentrated hydrochloric acid is one of the strongest acids and thus any desired pH from 0 to 7 can be easily achieved with the required dosage. Hydrochloric acid is seldom used in mineral flotation. The largest use is in hydrometallurgical processes and the pickling of hot rolled steel. In some cases, hydrochloric acid is used for decoating iron-stained mineral surfaces before flotation.Materials Uses
Piping, valves, and other equipment used in direct contact with anhydrous hydrogen chloride should be of stainless steel or of cast or mild steel. Carbon steel may be used in some components, but only if their temperature is controlled to remain below about 265°F (l29°C). In the presence of moisture, however, hydrogen chloride will corrode most metals. Teflon, Kel F and Hastelloy will resist corrosion.Smaller sized valves, such as those used on cylinders, constructed of aluminum-siliconbronze with Monel stems have had satisfactory service experience due to frequent maintenance. The satisfactory extension of these materials to other applications should be confirmed by testing prior to use.
Safety
When used diluted, at low concentration, hydrochloric acid is not usually associated with any adverse effects. However, the concentrated solution is corrosive and can cause severe damage on contact with the eyes and skin, or if ingested.LD50 (mouse, IP): 1.4 g/kg
LD50 (rabbit, oral): 0.9 g/kg
Potential Exposure
Hydrogen chloride itself is used in themanufacture of pharmaceutical hydrochlorides, chlorine, vinylchloride from acetylene; alkyl chlorides from olefins; arsenictrichloride from arsenic trioxide; in the chlorination of rubber;as a gaseous flux for babbitting operations; and in organic syn-thesis involving isomerization, polymerization, alkylation, andnitration reactions. The acid is used in the production of fertili-zers,dyes, dyestuffs, artificial silk, and paint pigments; inrefining edible oils and fats; in electroplating; leather tanning;ore refining; soap refining; petroleum extraction; pickling ofmetals; and in the photographic, textile, and rubber industries.It has been used as a choking/pulmonary agent.Physiological effects
ACGIH recommends a Threshold Limit ValueCeiling (TLV-C) of 5 ppm (7.5 mg/m3) for hydrogen chloride. The TLV-C is the concentration that should not be exceeded during any part of the working exposure.OSHA lists a Ceiling Value of 5 ppm (7 mg/m3) for hydrogen chloride. The Ceiling Value is the exposure limit that shall not be exceeded at any time during the working day. If instantaneous monitoring is not feasible, then the ceiling shall be assessed as a I5-minute TWA exposure that shall not be exceeded at any time during the working day [3].
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irri gate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, includ-ing resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medi-cal attention. If victim is conscious, administer water ormilk. Do not induce vomiting. Medical observation isrecommended for 24- -48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmo-nary edema, a doctor or authorized paramedic may consideradministering a corticosteroid spray. If frostbite hasoccurred, seek medical attentionimmediately; do NOT rubthe affected areas or flush them with water. In order to pre-vent further tissue damage, do NOT attempt to remove fro-zen clothing from frostbitten areas. If frostbite has NOToccurred, immediately and thoroughly wash contaminatedskin with soap and water.storage
Hydrochloric acid should be stored in a well-closed, glass or other inert container at a temperature below 30°C. Storage in close proximity to concentrated alkalis, metals, and cyanides should be avoided.storage
Splash goggles and rubber gloves should be worn when handling this acid, and containers of HCl should be stored in a wellventilated location separated from incompatible metals. Water should never be added to HCl because splattering may result; always add acid to water. Containers of hydrochloric acid should be stored in secondary plastic trays to avoid corrosion of metal storage shelves due to drips or spills.Cylinders of hydrogen chloride should be stored in cool, dry locations separated from alkali metals and other incompatible substances.
Shipping
Anhydrous hydrogen chloride requires a shippinglabel of“POISON GAS, CORROSIVE." It falls in HazardClass 2.3. It is a violation of transportation regulations torefill compressed gas cylinders without the express writtenpermission of the owner. Hydrogen chloride, refrigeratedliquid, requires a shipping label of“POISON GAS,CORROSIVE.”It falls in Hazard Class 8. Hydrochloricacid requires a shipping label of“CORROSIVE." It falls in Hazard Class 8 and Packing Group I. Procedures for thehandling, use, and storage of cylinders should be in compli-ance with OSHA 1910.101 and 1910.169 with the recom-mendations of the Compressed Gas Association.Special precautions: Cylinders must be transported in asecure upright position, in a well-ventilated truck.Purification Methods
Pass it through conc H2SO4, then over activated charcoal and silica gel. It fumes in moist air. Hydrogen chloride in gas cylinders contains ethylene, 1,1-dichloroethane and ethyl chloride. The latter two may be removed by fractionating the HCl through a trap cooled to -112o. Ethylene is difficult to remove. HCl fumes in moist air. HARMFUL VAPOURS. Its solubility in H2O is 82% at 0o. A constant boiling aqueous solution (azeotrope) has b 108.6o/760mm with an HCl concentration of ~20%, and is called Hydrochloric acid (muriatic acid) (see above). [Schmeisser in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 280-282 1963.]Toxicity evaluation
HCl causes local pH changes and denatures proteins. This leads to edema formation and tissue necrosis. HCl produces a coagulation necrosis characterized by the formation of a scar. Ingested HCl may give rise to damage of the esophagus and stomach. Gastric damage may occur secondary to pooling of HCl in the antrum as a result of pylorospasm. Patients who survive ingestions of HCl may develop stricture formation, gastric atony, and gastric outlet obstruction. When inhaled, HCl typically deposits in the upper respiratory tract and causes damage. Concentrated HCl can penetrate to the level of the bronchioles and alveoli and cause subsequent damage to these regions.Incompatibilities
Hydrochloric acid reacts violently with alkalis, with the evolution of a large amount of heat. Hydrochloric acid also reacts with many metals, liberating hydrogen.Incompatibilities
Hydrochloric acid and hydrogen chloride react violently with many metals, with the generation of highly flammable hydrogen gas, which may explode. Reaction with oxidizers such as permanganates, chlorates, chlorites, and hypochlorites may produce chlorine or bromine.Toxics Screening Level
There are two initial threshold screening levels (ITSL) for hydrogen chloride: 20 μg/m3 with an annual averaging time and 2,100 μg/m3 with a 1-hr averaging time.Waste Disposal
In many localities, hydrochloric acid or the residue from a spill may be disposed of down the drain after appropriate dilution and neutralization. Otherwise, hydrochloric acid and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines. Excess hydrogen chloride in cylinders should be returned to the manufacturer. For more information on disposal procedures, see Chapter 7 of this volume.Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (dental solutions; epidural injections; IM, IV, and SC injections; inhalations; ophthalmic preparations; oral solutions; nasal, otic, rectal, and topical preparations). Included in parenteral and nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.GRADES AVAILABLE
Anhydrous hydrogen chloride is typically available for commercial and industrial purposes in a technical grade (minimum purity of 99.0 percent).Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
- (E)-2-(2-Nitroethenyl)thiophene
- Pigment Yellow 14
- 2-Chloroethyl chloroformate
- 4,6-DIMETHOXYPYRIMIDINE-2-CARBOXYLIC ACID
- 1,2,3,4-THIATRIAZOL-5-AMINE
- 4-Nitrophenyl isocyanate
- 3-Phenoxypropionic acid
- 2-(2-Chloroethoxy)ethanol
- Potassium clavulanate
- 2-Chloro-3,4-diaminopyridine
- 1,3-Dichloro-5,5-dimethylhydantoin
- C^{12^} fatty alcohol polyoxyethylene ether ammonium sulfate
- trans-Ferulic acid
- 4,6-DIMETHYL-2-THIOPYRIMIDINE
- tert-Butylferrocene
- Ethylenediamine dihydrochloride
- 2-FLUOROPHENETHYL ALCOHOL
- 2,4-Dimethoxybenzoic acid
- Rubber peptizer
- 1,2,4,5-Tetrafluorobenzene
- 3-Hydroxy-2-iodo-6-methylpyridine
- 1H-Benzimidazole-5-carboxylic acid
- METHYLENEDIPHOSPHONIC ACID
- STRONTIUM HYDROXIDE OCTAHYDRATE
- Sodium 2,3-dihydroxynaphthalene-6-sulfonate
- Daidzein
- 4-METHOXY-3-METHYLBENZYL CHLORIDE
- QUINUCLIDINE HYDROCHLORIDE
- 4-NITROISOPHTHALIC ACID
- 4-CARBOXYPHENYL ISOTHIOCYANATE
1of8
7647-01-0, Hydrochloric acidRelated Search
1of4
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine