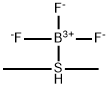
Description
Boron trifluoride methyl sulfide complex is reagent for the cleavage of benzyl ethers and carbamates.
Uses
Boron trifluoride methyl sulfide complex is a common reagent used for dealkylation of ethers. It can also be used to synthesize boron trifluoride complex with phosphine ligands.
Preparation
Boron trifluoride methyl sulfide complex is made by the addition of equimolar amounts of the two components at -78 °C. Dissociation of the complex reported as 96.2% at 12 °C and 97.4% at 34 °C. Also formed when crystalline bromodimethylsulfonium tetrafluoroborate is aspirated at rt to give a colorless product without change in crystal form. The reagent system is normally assembled by adding a large excess of dimethyl sulfide to an excess of boron trifluoride etherate.
reaction suitability
core: boron
reagent type: Lewis acid
reagent type: catalyst
storage
moisture-sensitive; use with carefully dried apparatus. All work must be carried out in an efficient fume hood.