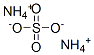Chemical Properties
Ammonium sulfate pure product is white crystal, and mostly appears slightly yellow when there are a small amount of impurities. Its physical properties are good, non-hygroscopic and non-caking. However, if too much sulfuric acid is added during the manufacturing process or the ambient humidity is high, it will absorb moisture and agglomerate. Ammonium sulfate is easily soluble in water, and the fertilizer aqueous solution exhibits an acidic reaction. It has stable chemical properties and does not volatilize or decompose under normal temperature and pressure. Under alkaline conditions, ammonia volatilization occurs and nitrogen is lost.
Application
Agricultural field: Ammonium sulfate is one of the indispensable nutritional elements for plant growth. It participates in the synthesis of chlorophyll and cell protein and has a significant impact on the growth, development and yield of crops. Applying ammonium sulfate can improve soil quality, increase soil microbial activity, and increase soil organic matter content. Ammonium sulfate can significantly increase crop yield and quality. It can increase the content of protein, vitamins and other nutrients in crops by providing sufficient nitrogen sources, thereby improving the nutritional value of crops. When used in appropriate amounts, ammonium sulfate can also promote plant growth and development and improve plant stress resistance.
Industrial field: Ammonium sulfate can also be used as a chemical raw material and is widely used in chemical industry, metallurgy and other industries. For example, it can be used to produce chemicals such as dyes and synthetic resins. Additionally, certain chemical properties of ammonium sulfate, such as its acidic nature, make it useful in laboratories to adjust the pH of solutions.
Production Methods
Ammonium sulphate is obtained by neutralizing ammonium hydroxide and sulfuric acid, crystallizing, centrifuging and drying. In the neutralization method, ammonia and sulfuric acid undergo a neutralization reaction at approximately 100°C. The resulting ammonium sulfate crystal slurry is centrifuged and dried to obtain ammonium sulfate finished product.
2NH3+H2SO4→(NH4)2SO4
Its recovery method is obtained by recovering ammonia from coke oven gas and then neutralizing it with sulfuric acid.
Agricultural Uses
Try not to apply ammonium sulfate to paddy fields. Because under flooded conditions, the soil will be severely oxygen-deficient, after ammonium sulfate is applied, the sulfate ions in it will be reduced to sulfide. Excessive accumulation of sulfide concentration can cause plant root damage and death.
It is not advisable to apply ammonium sulfate on the same cultivated land for a long time, otherwise the soil will become acidic and cause hardening. If it is really necessary to apply, some lime or organic fertilizer can be applied in appropriate amounts. However, it must be noted that ammonium sulfate and lime cannot be applied together to prevent ammonium sulfate from decomposing and causing nitrogen loss. Generally, the combined application of the two should be separated by 3 to 5 days.
Properties and Applications
Ammonium sulfate fertilizer is rich in nitrogen and can provide nutrients needed for plant growth. Nitrogen is an important component for plants to synthesize protein and chlorophyll, and is crucial to plant growth and development.
At the same time, ammonium sulfate fertilizer also contains sulfur, which is one of the necessary nutrients for plants. Sulfur is involved in the synthesis and activity regulation of various enzymes in plants, and plays an important role in regulating photosynthesis and nutrient absorption of plants.